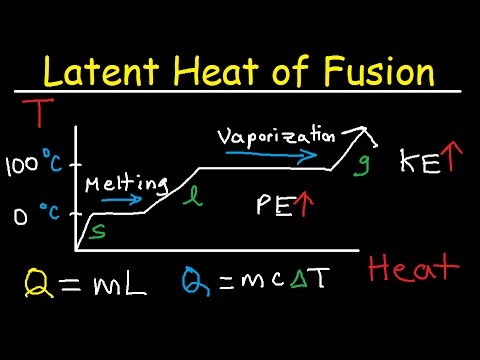
Similarly, while ice melts, it remains at 0 °C (32 °F), and the liquid water that is formed with the latent heat of fusion is also at 0 °C. The heat of fusion for water at 0 °C is approximately 334 joules (79.7 calories) per gram, and the heat of vaporization at 100 °C is about 2,230 joules (533 calories) per gram.
Q. What happens when heat is supplied to a solid substance?
When heat is supplied to a solid substance, the kinetic energy of the particles increases. This is because, as the heat is absorbed by the particles, kinetic energy becomes more. and this increase causes the particles to move away. this phenomenon is also responsible for change in physical state.
Table of Contents
Q. What do you understand by latent heat of fusion?
The amount of heat required to convert one unit amount of substance from the solid phase to the liquid phase — leaving the temperature of the system unaltered — is known as the latent heat of fusion.
Q. On what factors does the Vapour pressure of a liquid depends?
Vapor pressure is the pressure caused by the evaporation of liquids. Three common factors that influence vapor press are surface area, intermolecular forces and temperature. The vapor pressure of a molecule differs at different temperatures.
Q. Does boiling depend on surface area?
Branching decreases the boiling point So the increase of surface area increases the ability of individual molecules to attract each other. Branching in molecules decreases the surface area thereby decreasing the attractive force between individual molecules. As a result, the boiling point decreases.
Q. Does pressure depend on surface area?
Pressure as a Function of Surface Area Since pressure depends only on the force acting perpendicular to the surface upon which it is applied, only the force component perpendicular to the surface contributes to the pressure exerted by that force on that surface.
Q. How can we increase Vapour pressure?
As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.