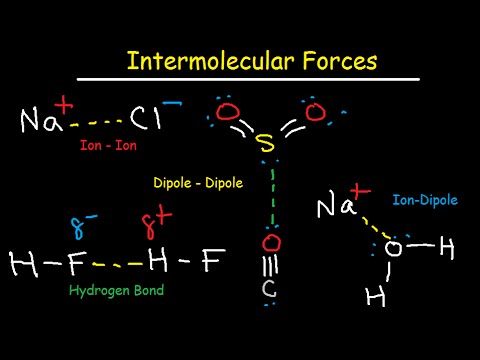
Q. What kind of force is present in ionic bonds?
electrostatic forces
Q. What is an ionic bond simple definition?
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
Table of Contents
- Q. What kind of force is present in ionic bonds?
- Q. What is an ionic bond simple definition?
- Q. Are ionic bonds positive or negative?
- Q. How do you use ionic bonding in a sentence?
- Q. What are 3 characteristics of ionic bonds?
- Q. What is ionic bond and its characteristics?
- Q. What are the basic features of ionic and covalent bond?
- Q. What are the 2 examples of ion?
- Q. What does kno mean in text?
- Q. What is kno full form?
- Q. What does ION mean in Snapchat?
- Q. What mean knot?
- Q. How much money is a knot?
- Q. What is the example of knot?
- Q. What is the purpose of the knot?
- Q. What is the strongest knot?
- Q. What is the best knot?
- Q. What is the best stopper knot?
- Q. What are the 3 stopper knots?
- Q. What is a dead knot?
Q. Are ionic bonds positive or negative?
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
Q. How do you use ionic bonding in a sentence?
Use “ionic bond” in a sentence | “ionic bond” sentence examples
- Ionic bonds form most strongly between elements like sodium and chlorine.
- The microparticle could link with pulp fibre through ionic bond and hydrogen bond, from which excellent retention effect of copolymer initiated.
Q. What are 3 characteristics of ionic bonds?
Properties Shared by Ionic Compounds
- They form crystals.
- They have high melting points and high boiling points.
- They have higher enthalpies of fusion and vaporization than molecular compounds.
- They’re hard and brittle.
- They conduct electricity when they are dissolved in water.
- They’re good insulators.
Q. What is ionic bond and its characteristics?
Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. An ionic compound can be identified by its chemical formula: metal + nonmetal or polyatomic ions.
Q. What are the basic features of ionic and covalent bond?
PROPERTIES OF IONIC COMPOUNDS
| Ionic compounds | Covalent compounds |
|---|---|
| They have high melting points and boiling points. That is, ionic compounds are non-volatile. | They have usually low melting points and boiling points. That is, covalent compounds are usually volatile. |
Q. What are the 2 examples of ion?
An ion is a positively or negatively charged atom (or group of atoms). An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons. Example: Sodium ion Na+, magnesium ion Mg2+, chloride ion Cl–, and oxide ion O2–.
Q. What does kno mean in text?
Know
Q. What is kno full form?
The Full form of KNO is know, or KNO stands for know, or the full name of given abbreviation is know.
Q. What does ION mean in Snapchat?
In Other News
Q. What mean knot?
(Entry 1 of 3) 1a : an interlacement of the parts of one or more flexible bodies forming a lump or knob (as for fastening or tying together) b : the lump or knob so formed. c : a tight constriction or the sense of constriction my stomach was all in knots.
Q. How much money is a knot?
Definitions include: money. Definitions include: one thousand dollars.
Q. What is the example of knot?
The definition of a knot is the looping and tying of a piece of string or rope, or the place where a tree limb joins the trunk. An example of a knot is a tied shoelace. An example of a knot is the cross-grained circular part on some boards.
Q. What is the purpose of the knot?
The knot is used to attach a rope to a ring, hook, anchor, or other object. It is made by taking two rounds of the rope around a solid object, then passing the end under both turns to form a pair of half hitches.
Q. What is the strongest knot?
Palomar knot
Q. What is the best knot?
5 Best Knots to Know
- Bowline Knot. Anna.zabella/Shutterstock. Use this knot to tie super-strong, non-jamming loops.
- Square Knot. Anna.zabella/Shutterstock.
- Sheet Bend. Anna.zabella/Shutterstock.
- Double Half Hitches. Anna.zabella/Shutterstock.
Q. What is the best stopper knot?
Figure Eight Stopper Knot
Q. What are the 3 stopper knots?
To pull it out is to unreeve it. Stopper knots prevent the rope from unreeving on its own. “Stopper” has three distinct meanings in the context of knotting and cordage. A decorative stopper knot may be referred to as a lanyard knot….
| Stopper knot | |
|---|---|
| Typical use | Keeps the line from slipping out of things. |
Q. What is a dead knot?
A knot that has lost its fibrous connection with the surrounding wood; it can easily loosen and fall out or be knocked out.