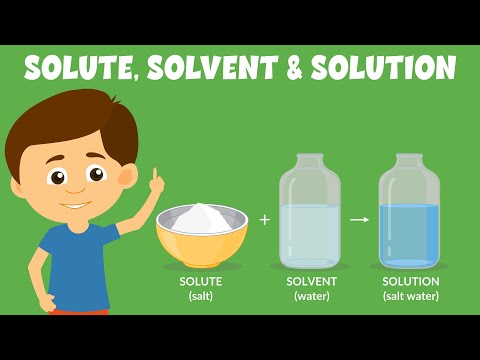
Q. What liquids are solutions?
Solutions can be solids dissolved in liquids. When you work with chemistry or even cook in your kitchen, you will usually be dissolving solids into liquids. Solutions can also be gases dissolved in liquids, such as carbonated water. There can also be gases in other gases and liquids in liquids.
Q. What makes up most of a solution?
A solution is a homogeneous type of mixture of two or more substances. A solution has two parts: a solute and a solvent. The solute is the substance that dissolves, and the solvent is the majority of the solution.
Table of Contents
- Q. What liquids are solutions?
- Q. What makes up most of a solution?
- Q. Are all solution in liquid form?
- Q. What are the solution of gas in a liquid?
- Q. Can liquid dissolve gas?
- Q. What gases can be dissolved in water?
- Q. Which gas is more soluble in liquid?
- Q. Which is more soluble in water?
- Q. What are gas solutions examples?
- Q. What are the 10 example of solution?
- Q. Which is the best example of a solution?
- Q. What are the 2 components of solution?
- Q. What are parts of solution?
- Q. What are characteristics of solution?
- Q. What is a pure solution?
- Q. Is Hot Tea a mixture?
Q. Are all solution in liquid form?
In all solutions, whether gaseous, liquid, or solid, the substance present in the greatest amount is the solvent, and the substance or substances present in lesser amounts are the solute(s)….13.2: Types of Solutions and Solubility.
| Solution | liquid |
|---|---|
| Solute | liquid |
| Solvent | liquid |
| Examples | alcoholic beverage (ethanol in water), gasoline |
Q. What are the solution of gas in a liquid?
Solutions having solute in gaseous state and solvent in liquid state, are called Gas – Liquid Solutions. For example – Solution (mixture) of oxygen in water, mixture of carbon dioxide in water. Coca cola, a beverage, is an example of gas – liquid solution, as it has carbon dioxide dissolved in water.
Q. Can liquid dissolve gas?
Gases dissolve in liquids to form solutions. This dissolution is an equilibrium process for which an equilibrium constant can be written. For example, the equilibrium between oxygen gas and dissolved oxygen in water is O2(aq) <=> O2(g).
Q. What gases can be dissolved in water?
The main gases dissolved in purified water are oxygen and nitrogen, carbon dioxide, plus traces of inert gases, all in equilibrium with ambient air.
Q. Which gas is more soluble in liquid?
CO2 is soluble because water molecules are attracted to these polar areas. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water.
Q. Which is more soluble in water?
Among given compounds, ethylene glycol ( HO−CH2−CH2−OH ) is the most soluble in water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water.
Q. What are gas solutions examples?
13.1: Types of Solutions – Some Terminology
| Solution | Solute | Examples |
|---|---|---|
| gas | gas | air, natural gas |
| liquid | gas | seltzer water (CO2 gas in water) |
| liquid | liquid | alcoholic beverage (ethanol in water), gasoline |
| liquid | solid | tea, salt water |
Q. What are the 10 example of solution?
bleach (sodium hypochlorite dissolved in water) dishwater (soap dissolved in water) carbonated beverages (carbon dioxide dissolved in water is what gives sodas their fizz) powdered drinks.
Q. Which is the best example of a solution?
The best example of a solution is seawater since seawater is a mixture of both salt and water mixed together.
Q. What are the 2 components of solution?
Components of a Solution It has basically has two components i.e. a solvent and a solute. Solvent: The component of a solution which dissolves the other component in itself is called solvent. A solvent constitutes the larger component of the solution.
Q. What are parts of solution?
All solutions have two parts: the solute and the solvent. The solute is the substance that dissolves, and the solvent is the substance that dissolves the solute.
Q. What are characteristics of solution?
A solution is a homogeneous mixture of two or more substances. The particles of solute in a solution cannot be seen by the naked eye. A solution does not allow beams of light to scatter. A solution is stable.
Q. What is a pure solution?
Pure Solution – Pure sultion means a solution which does not contain dissolved imputities in it.
Q. Is Hot Tea a mixture?
A Tea is a solution of compounds in water, so it is not chemically pure. It is usually separated from tea leaves by filtration. B Because the composition of the solution is uniform throughout, it is a homogeneous mixture.