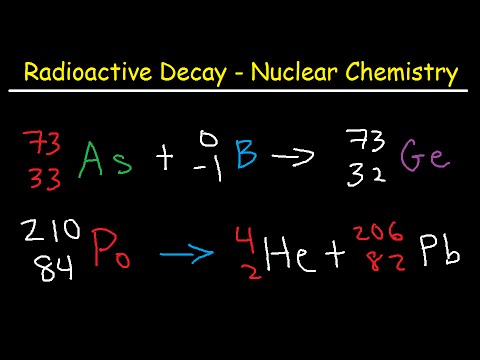
Q. What particles are emitted during radioactive decay?
The nature of radioactive emissions The emissions of the most common forms of spontaneous radioactive decay are the alpha (α) particle, the beta (β) particle, the gamma (γ) ray, and the neutrino.
Q. What are the names of the three types of particles that get emitted during radioactive decay?
There are many types of emmitted particles and radiation that radioisotopes produce when they decay. The types we will discuss here are: alpha, beta, and gamma (listed in increasing ability to penetrate matter). Alpha decay is seen only in heavier elements greater than atomic number 52, tellurium.
Table of Contents
- Q. What particles are emitted during radioactive decay?
- Q. What are the names of the three types of particles that get emitted during radioactive decay?
- Q. What are alpha and beta particles?
- Q. What are the possible particles emitted in the decay process?
- Q. How do you determine if the decay is an Alpha?
- Q. How do you identify radioactivity?
- Q. What is the daughter nuclide?
- Q. What is a parent nuclide?
- Q. What changes the rate of radioactive decay?
- Q. What is the measure of radioactive decay rate?
- Q. Is there a way to speed up radioactive decay?
- Q. What is rate of decay?
- Q. What’s the meaning of exponential?
- Q. What is another word for exponential?
- Q. What’s the opposite of exponential?
- Q. Why do we use exponential?
- Q. What is the difference between logarithmic and exponential?
- Q. What is log and exponential?
- Q. How do you convert exponential to log?
- Q. How do you know if a relationship is exponential?
- Q. Why do we use natural log in finance?
Q. What are alpha and beta particles?
Alpha radiation is the name for the emission of an alpha particle in fact an helium nuclei, beta radiation is the emission of electrons or positrons , and gamma radiation is the term used for the emission of energetic photons.
Q. What are the possible particles emitted in the decay process?
In the different radioactive decay processes, α particles (4He nuclei), β particles (electrons), and γ rays (high-energy photons) are emitted.
Q. How do you determine if the decay is an Alpha?
So first look at the father nucleus and list its number of protons and its atomic weight. Step 3) Now from number of neutrons subtract 2 and from number of protons subtract 2 as an alpha particle has 2 neutrons and 2 protons and in an alpha decay an alpha particle will always form in case of any any father nucleus.
Q. How do you identify radioactivity?
To determine the type of radiation (alpha, beta or gamma), first determine the background count rate, then the source count rate with no absorber. Next, place a sheet of paper between the source and the monitor. If the counts are significantly reduced, the source emits alpha particles.
Q. What is the daughter nuclide?
daughter nuclide: a nuclide produced by the radioactive decay of another nuclide. May be stable or may decay further.
Q. What is a parent nuclide?
A parent nuclide is a nuclide that decays into a specific daughter nuclide during radioactive decay. A parent nuclide is also known as a parent isotope.
Q. What changes the rate of radioactive decay?
The half-life of radioactive decay can also be altered by changing the state of the electrons surrounding the nucleus. In a type of radioactive decay called “electron capture”, the nucleus absorbs one of the atom’s electrons and combines it with a proton to make a neutron and a neutrino.
Q. What is the measure of radioactive decay rate?
Curies
Q. Is there a way to speed up radioactive decay?
Atoms of beryllium-7 decay by grabbing electrons from their surroundings. The rate of this kind of decay depends on the chance of an electron straying into the nucleus and getting absorbed. So increasing the density of electrons surrounding the atomic nucleus can speed up the decay.
Q. What is rate of decay?
The rate of decay, or activity, of a sample of a radioactive substance is the decrease in the number of radioactive nuclei per unit time.
Q. What’s the meaning of exponential?
1 : of or relating to an exponent. 2 : involving a variable in an exponent 10x is an exponential expression. 3 : expressible or approximately expressible by an exponential function especially : characterized by or being an extremely rapid increase (as in size or extent) an exponential growth rate.
Q. What is another word for exponential?
What is another word for exponential?
| aggressive | epidemic |
|---|---|
| ascending | augmented |
| expanding | growing |
| mounting | rampant |
| rapid change | rapid growth |
Q. What’s the opposite of exponential?
Logarithmic growth
Q. Why do we use exponential?
Introduction. Exponential functions can be used to model growth and decay. Exponential functions are ever-increasing so saying that an exponential function models population growth exactly means that the human population will grow without bound.
Q. What is the difference between logarithmic and exponential?
The exponential function is given by ƒ(x) = ex, whereas the logarithmic function is given by g(x) = ln x, and former is the inverse of the latter. The domain of the exponential function is a set of real numbers, but the domain of the logarithmic function is a set of positive real numbers.
Q. What is log and exponential?
Logarithmic functions are the inverses of exponential functions. The inverse of the exponential function y = ax is x = ay. The logarithmic function y = logax is defined to be equivalent to the exponential equation x = ay. This unknown exponent, y, equals logax. So you see a logarithm is nothing more than an exponent.
Q. How do you convert exponential to log?
To change from exponential form to logarithmic form, identify the base of the exponential equation and move the base to the other side of the equal sign and add the word “log”. Do not move anything but the base, the other numbers or variables will not change sides.
Q. How do you know if a relationship is exponential?
Linear and exponential relationships differ in the way the y-values change when the x-values increase by a constant amount:
- In a linear relationship, the y-values have equal differences.
- In an exponential relationship, the y-values have equal ratios.
Q. Why do we use natural log in finance?
We prefer natural logs (that is, logarithms base e) because, as described above, coefficients on the natural-log scale are directly interpretable as approximate proportional differences: with a coefficient of 0.06, a difference of 1 in x corresponds to an approximate 6% difference in y, and so forth.