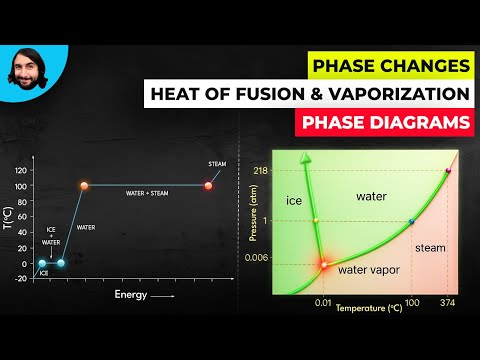
Q. What phase change happens in the process of vaporization?
Vaporization, conversion of a substance from the liquid or solid phase into the gaseous (vapour) phase. If conditions allow the formation of vapour bubbles within a liquid, the vaporization process is called boiling. Direct conversion from solid to vapour is called sublimation.
Q. What are the three main processes of water cycle?
The water cycle is often taught as a simple circular cycle of evaporation, condensation, and precipitation.
Table of Contents
- Q. What phase change happens in the process of vaporization?
- Q. What are the three main processes of water cycle?
- Q. Can gas turn into solid without being liquid?
- Q. What are three examples of sublimation?
- Q. What is the real life example of sublimation?
- Q. What freezing point means?
- Q. What prevents ice from forming?
Q. Can gas turn into solid without being liquid?
Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation.
Q. What are three examples of sublimation?
To help you gain a better understanding of this process, here are some real-life examples of sublimation:
- Dry Ice. As mentioned earlier, dry ice is one of the most popular examples of sublimation in real life.
- Water.
- Specialized Printers.
- Moth Balls.
- Freeze Drying.
- Air Fresheners.
Q. What is the real life example of sublimation?
Dry ice, Solid Iodine, and Ammonium Chloride are examples of Sublimation. It is a much less frequent transformation of matter than evaporation or fusion, which usually requires the injection of caloric energy until reaching a variable point according to the nature of the matter, called sublimation point.
Q. What freezing point means?
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure. Alternatively, a melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
Q. What prevents ice from forming?
The typical approach to clearing off the ice is to use a deicing solution once the ice has built up. The fluid created by the Ames team, though, when applied to a dry surface, prevents the ice from even forming a surface bond, which saves deicing time and money, while also preventing excessive use of chemical solvents.