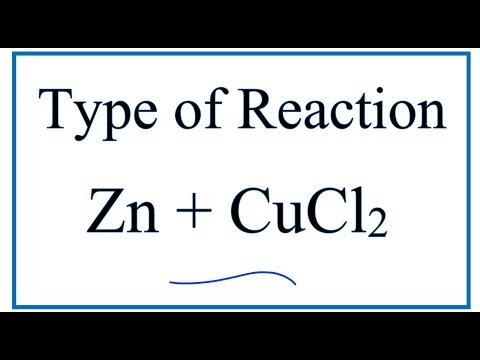
Q. What type of reaction is ZnCl2?
single replacement reaction
Q. Is Zinc made of compounds?
In chemical compounds, zinc exhibits almost exclusively a +2 oxidation state. A few compounds of zinc in the +1 state have been reported, but never any compounds of zinc in the +3 state or higher. Zinc oxide, ZnO, is one of the most important zinc compounds.
Table of Contents
- Q. What type of reaction is ZnCl2?
- Q. Is Zinc made of compounds?
- Q. Why is zinc used in paint?
- Q. Why is Iron less reactive than zinc?
- Q. Is Iron reactive than zinc?
- Q. Why Aluminium is more reactive than zinc?
- Q. Which metal is reactive iron or zinc?
- Q. Which metal is more reactive Aluminium or zinc?
- Q. Is cadmium more reactive than zinc?
- Q. Is magnesium more reactive than lithium?
Q. Why is zinc used in paint?
One of the most important ingredients is Zinc Oxide. It used primarily in primers and exterior paints, Zinc Oxide provides mildew resistance, corrosion inhibition, and stain blocking support. In the case of linseed oil, the formation of zinc soaps from zinc oxide tends to harden the paint film.
Q. Why is Iron less reactive than zinc?
Zinc displaces copper, and iron from their solutions. Zinc is more reactive than Cu and Fe metal. Iron displaces copper from its solution. Copper does not displace any metal therefore it is the least reactive.
Q. Is Iron reactive than zinc?
In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom….The reactivity series.
| Element | Reaction with dilute acids |
|---|---|
| Iron | More slowly than zinc |
| Copper | Very slowly |
| Silver | Barely reacts |
| Gold | Does not react |
Q. Why Aluminium is more reactive than zinc?
Because aluminum replaces compounds of zinc, iron, and copper to produce pure zinc, iron, and copper. Aluminum reacts more. Look how pure aluminum rips the chlorine away from the zinc chloride to produce aluminum chloride and zinc.
Q. Which metal is reactive iron or zinc?
Answer : Zinc is more reactive than iron. Zinc is placed above iron in the reactivity series. For example, Zinc can displace iron from ferrous sulphate solution to form zinc sulphate and iron.
Q. Which metal is more reactive Aluminium or zinc?
So aluminium is more reactive than zinc. If small pieces of copper are added into solutions of zinc sulphate, aluminium sulphate and ferrous sulphate, no reactions take place. So, copper is less reactive than zinc, aluminium and iron.
Q. Is cadmium more reactive than zinc?
For example, magnesium metal can displace zinc ions in a solution. Mg(s) + Zn2+ →→ Zn(s) + Mg….Reactivity Series of metals Chart.
| Metal | Cadmium |
|---|---|
| Symbol | Cd |
| Reactivity | Displaces H2 gas from acids only and forms hydroxides. |
| Extraction | Smelting with coke |
Q. Is magnesium more reactive than lithium?
Why magnesium is less reactive than lithium – Physics Stack Exchange.