Q. What was wrong with Democritus atomic theory?
2,500 years ago, Democritus suggested that all matter in the universe was made up of tiny, indivisible, solid objects he called “atomos.” However, other Greek philosophers disliked Democritus’ “atomos” theory because they felt it was illogical. Atoms of different elements differ in size, mass, and other properties.
Q. What did Democritus believe an atom was?
Around 400 B.C.E., the Greek philosopher Democritus introduced the idea of the atom as the basic building block matter. Democritus thought that atoms are tiny, uncuttable, solid particles that are surrounded by empty space and constantly moving at random.
Table of Contents
- Q. What was wrong with Democritus atomic theory?
- Q. What did Democritus believe an atom was?
- Q. Why Democritus could not test his ideas about the atom Why do you think we are able to study the atom in the modern world?
- Q. What was Democritus’s theory?
- Q. What was Democritus full name?
- Q. Did Democritus believe in God?
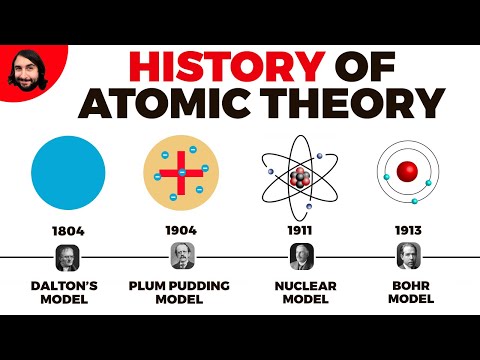
- Q. What was the Dalton model?
- Q. What were Daltons 5 main points?
- Q. What are Daltons 4 theories?
- Q. What was Ernest Rutherford’s experiment?
- Q. What are the two main features of Rutherford’s atomic model?
- Q. Why was Rutherford model wrong?
- Q. What were two conclusions of the gold foil experiment?
- Q. Which conclusion is based on the gold foil experiment?
- Q. What three conclusions came from the gold foil experiment?
- Q. Why did Rutherford use gold instead of magnesium foil?
- Q. What did Rutherford’s famous scattering experiment prove in his day?
- Q. What is the alpha scattering experiment?
- Q. How close does the alpha particle get to the gold nucleus before turning around?
- Q. Why did some of the alpha particles rebound?
Q. Why Democritus could not test his ideas about the atom Why do you think we are able to study the atom in the modern world?
Explain why Democritus was unable to experimentally verify his ideas. Democritus was unable to explain his ideas because he didn’t have the resources he need to prove the existence of atoms. Aristotle believed that there was no empty space, therefore atoms could not move through empty space.
Q. What was Democritus’s theory?
Democritus was a central figure in the development of the atomic theory of the universe. He theorized that all material bodies are made up of indivisibly small “atoms.” Aristotle famously rejected atomism in On Generation and Corruption.
Q. What was Democritus full name?
Democritus (Greek: Δημόκριτος) was an ancient Greek philosopher. He was born in Thrace, Greece, circa 460 BC. He was a rich citizen of Abdera, in Thrace, and a student of Leucippus, another Greek philosopher.
Q. Did Democritus believe in God?
Democritus did not believe in God or the gods, believing the world to be governed entirely by natural laws. Some ancient sources claim he lived longer: the third century AD Greek biographer Diogenes Laërtius said Democritus lived for 109 years.
Q. What was the Dalton model?
Dalton’s atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass.
Q. What were Daltons 5 main points?
Terms in this set (5)
- Elements are composed of extremely small particles called atoms that are indivisible and indestructible.
- All atoms of a given element are identical; they have the same size, mass, and chemical properties.
- Atoms of 1 element are different from the atoms of all other elements.
Q. What are Daltons 4 theories?
1) All matter is made of atoms. Atoms are indivisible and indestructible. 3) Compounds are formed by a combination of two or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms.
Q. What was Ernest Rutherford’s experiment?
Ernest Rutherford’s most famous experiment is the gold foil experiment. A beam of alpha particles was aimed at a piece of gold foil. Most alpha particles passed through the foil, but a few were scattered backward. This showed that most of the atom is empty space surrounding a tiny nucleus.
Q. What are the two main features of Rutherford’s atomic model?
The salient features of this model are as follows: (i) The atom contains a central part called nucleus which is surrounded by electrons. (ii) The nucleus of an atom is positively charged. (iii) The size of the nucleus is very small as compared to the atomic size.
Q. Why was Rutherford model wrong?
The main problem with Rutherford’s model was that he couldn’t explain why negatively charged electrons remain in orbit when they should instantly fall into the positively charged nucleus. This problem would be solved by Niels Bohr in 1913 (discussed in Chapter 10).
Q. What were two conclusions of the gold foil experiment?
From the location and number of α-particles reaching the screen, Rutherford concluded the following: i) Almost 99% of the α-particles pass through the gold foil without any deflection. So atom must be having a lot of empty space in it. ii) Several α-particles get deflected at angles.
Q. Which conclusion is based on the gold foil experiment?
The gold foil experiment led to the conclusion that each atom in the foil was composed mostly of empty space because most alpha particles directed at the foil 1) An atom is mostly empty space with a dense, positively charged nucleus.
Q. What three conclusions came from the gold foil experiment?
Most of the alpha particles did pass straight through the foil. The atom being mostly empty space. A small number of alpha particles were deflected by large angles (> 4°) as they passed through the foil. There is a concentration of positive charge in the atom.
Q. Why did Rutherford use gold instead of magnesium foil?
Rutherford used gold for his scattering experiment because gold is the most malleable metal and he wanted the thinnest layer as possible. The gold sheet used was around 1000 atoms thick. Therefore, Rutherford selected a Gold foil in his alpha scatttering experiment.
Q. What did Rutherford’s famous scattering experiment prove in his day?
These experiments led Rutherford to describe the atom as containing mostly empty space, with a very small, dense, positively charged nucleus at the center, which contained most of the mass of the atom, with the electrons orbiting the nucleus.
Q. What is the alpha scattering experiment?
The atom was believed to consist of a positive material “pudding” with negative “plums” distributed throughout. Rutherford directed beams of alpha particles (which are the nuclei of helium atoms and hence positively charged) at thin gold foil to test this model and noted how the alpha particles scattered from the foil.
Q. How close does the alpha particle get to the gold nucleus before turning around?
An Alpha Particle, Initially Very Far From The Gold Nucleus, Is Fired At 1.9 107 M/s Directly Toward The Nucleus, As In The Figure Below.
Q. Why did some of the alpha particles rebound?
” Only a few particles bounced back because the nucleus is so small / atom is mostly empty space that few collided with a nucleus . If they bounced back it was because of alpha particle and nucleus are positive : repulsion if they bounced back it was because nucleus is massive / dense so alpha particles rebound . “