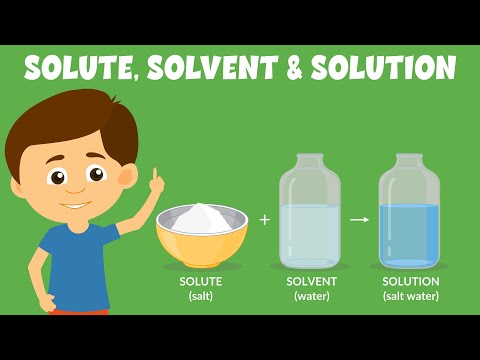
Q. Whats is a solution?
A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. solvent: the substance in which a solute dissolves to produce a homogeneous mixture. solute: the substance that dissolves in a solvent to produce a homogeneous mixture.
Q. What is solution and example?
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. Common examples of solutions are the sugar in water and salt in water solutions, soda water, etc. In a solution, all the components appear as a single phase.
Table of Contents
- Q. Whats is a solution?
- Q. What is solution and example?
- Q. How do you describe the solution?
- Q. What is a solution give two examples?
- Q. What are the 3 types of solutions?
- Q. What are the 9 types of solutions?
- Q. What are the 2 types of solutions?
- Q. What are the 5 example of solution?
- Q. What are the most common types of solutions?
- Q. What are the type of solutions?
- Q. What are normal solutions?
- Q. What is the importance of solutions?
- Q. What are the 3 importance of solution in our daily lives?
- Q. What are the components of solutions?
- Q. What makes coffee a solution?
- Q. Is tea a colloid suspension or solution?
- Q. Is orange juice a solution?
- Q. Is milk a solution?
- Q. Why is milk not a solution?
- Q. Is milk powder a solute?
- Q. Is blood a true solution?
- Q. What is blood a solution of?
- Q. Is tea a true solution?
- Q. Is Air is a true solution?
- Q. Which is a true solution?
- Q. What are examples of true solutions?
- Q. Is lemon juice a true solution?
- Q. Is Vinegar a true solution?
- Q. What is the other name of true solution?
- Q. Is sugar and water a true solution?
Q. How do you describe the solution?
A solution is a homogeneous mixture of two or more substances. The particles of solute in a solution cannot be seen by the naked eye. A solution does not allow beams of light to scatter. A solution is stable.
Q. What is a solution give two examples?
Some examples of solutions are salt water, rubbing alcohol, and sugar dissolved in water. When you look closely, upon mixing salt with water, you can’t see the salt particles anymore, making this a homogeneous mixture. Let’s make use of our salt water example to talk about the two main parts of a solution.
Q. What are the 3 types of solutions?
There are three types of solutions that can occur in your body based on solute concentration: isotonic, hypotonic, and hypertonic.
Q. What are the 9 types of solutions?
Terms in this set (9)
- Solid Solute (Liquid) vinegar.
- Liquid Solute (Liquid) Salt water.
- Gas Solute (Liquid) Soft drink.
- Solid Solute (Gas) Mothballs.
- Liquid (Gas) humidity.
- Gas Solute (Gas) air.
- Solid Solute (Solid) gold-silver.
- Liquid Solute (Solid) dental filings.
Q. What are the 2 types of solutions?
The solutions are of two forms, depending on whether the solvent is water or not.
- Aqueous solution – When a solute is dissolved in water the solution is called an aqueous solution.
- Non-aqueous solution – When a solute is dissolved in a solvent other than water, it is called a non-aqueous solution.
Q. What are the 5 example of solution?
bleach (sodium hypochlorite dissolved in water) dishwater (soap dissolved in water) carbonated beverages (carbon dioxide dissolved in water is what gives sodas their fizz) powdered drinks.
Q. What are the most common types of solutions?
Examples of Solutions in Everyday Life
- Liquid/Liquid Solutions. Many household liquids and automotive products are examples of liquid/liquid solutions.
- Solid/Liquid Solutions.
- Gas/Liquid Solutions.
- Gas/Gas Solutions.
- Solid/Solid Solutions.
- Solutions.
- Suspensions.
- Colloids.
Q. What are the type of solutions?
13.1: Types of Solutions – Some Terminology
| Solution | Solute | Examples |
|---|---|---|
| gas | gas | air, natural gas |
| liquid | gas | seltzer water (CO2 gas in water) |
| liquid | liquid | alcoholic beverage (ethanol in water), gasoline |
| liquid | solid | tea, salt water |
Q. What are normal solutions?
It is similar to molarity but uses the gram-equivalent weight of a solute in its expression of solute amount in a liter (L) of solution, rather than the gram molecular weight (GMW) expressed in molarity. A 1N solution contains 1 gram-equivalent weight of solute per liter of solution.
Q. What is the importance of solutions?
Answer: Solution is very important in the study of foods and human nutrition. Only substances which can be dissolved can be assimilated. Many substances which will not dissolve in pure water will dissolve in water which contains something else in solution.
Q. What are the 3 importance of solution in our daily lives?
Air. Many chemical reactions are carried out in solutions, and solutions are also closely related to our everyday lives. The air we breathe, the liquids we drink, and the fluids in our body are all solutions. Furthermore, we are surrounded by solutions such as the air and waters (in rivers, lakes and oceans).
Q. What are the components of solutions?
Solutions are made up of two parts: a solvent and a solute. Solvent – The component that dissolves the other component is called the solvent. Solute – The component that is dissolved in the solvent is called solute.
Q. What makes coffee a solution?
At a very basic level, brewed coffee is a solution which results from combining ground coffee beans and hot water. In this interaction, the coffee beans operate as a solute, or substance which is dissolved to create a solution, and hot water acts as a solvent, or the substance used to dissolve the solute.
Q. Is tea a colloid suspension or solution?
A mixture with particles that can be easily seen and separated by being filtered or settling. If you try to put too much sugar into a cup of tea, it will not fully dissolve and will leave some sugar on the bottom of the cup, making the tea a Suspension.
Q. Is orange juice a solution?
A homogeneous mixture often called as solution, orange juice is a solution without pulp, uniformly distributed throughout the mixture. Orange juice with pulp is not uniformly distributed through-out the mixture called heterogeneous mixture, often called as suspension.
Q. Is milk a solution?
Milk is not a solution because it has more than one phase suspended in it — it has a liquid phase and a solid phase. Unhomogenized milk is not a solution, it’s a suspension because the fat (aka cream) will separate from the rest of the milk and rise to the top, since fat is less dense than water.
Q. Why is milk not a solution?
Q. Is milk powder a solute?
Explanation: The solid material like soap, powdered juice, powdered milk, chocolate powder, and others which is being dissolve is called solute.
Q. Is blood a true solution?
True solution is a homogeneous mixture of two or more substances. Step by step solution: A true solution is a homogeneous mixture with uniform properties throughout. From the above explanation we can say that blood, ink, starch are colloidal solutions and sugar sol and salt sol are true solutions.
Q. What is blood a solution of?
The liquid portion of the blood, the plasma, is a complex solution containing more than 90 percent water. The water of the plasma is freely exchangeable with that of body cells and other extracellular fluids and is available to maintain the normal state of hydration of all tissues.
Q. Is tea a true solution?
A Tea is a solution of compounds in water, so it is not chemically pure. It is usually separated from tea leaves by filtration. B Because the composition of the solution is uniform throughout, it is a homogeneous mixture.
Q. Is Air is a true solution?
Air is considered a solution because it cannot be filtered, negative for the Tyndall effect and its components cannot be separated.
Q. Which is a true solution?
True Solution is a homogeneous mixture of two or more materials with a particle size of less than 10-9 m or 1 nm dissolved in the solvent. Particles can not be isolated from true solutions by using filter paper which is also not apparent to the naked eye.
Q. What are examples of true solutions?
True solution – Salt solution, copper sulphate solution, sugar and water solution, vinegar, air, brass. Colloidal solution – Milk, Blood, Soap solution, starch solution, ink.
Q. Is lemon juice a true solution?
Mixed together, lemon juice, water, and sugar form a solution. A solution is a mixture in which the different kinds of matter are spread out evenly. In lemonade, water is called the solvent. Sugar and lemon juice are solutes.
Q. Is Vinegar a true solution?
Yes, vinegar is a true solution.
Q. What is the other name of true solution?
The true solution is the homogenous mixture, while Colloidal solution and Suspension are the heterogeneous mixtures of two or more substances.
Q. Is sugar and water a true solution?
True Solution is a homogeneous mixture of two or more substances in which substance dissolved (solute) in solvent has the particle size of less than 10-9 m or 1 nm. Simple solution of sugar in water is an example of true solution.