1804
Q. What did John Dalton contribute to the understanding of the atom?
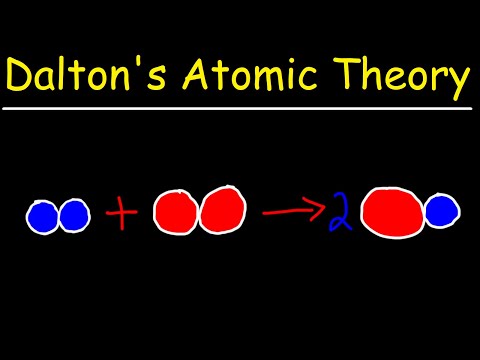
Dalton’s atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. Dalton also postulated that chemical reactions resulted in the rearrangement of the reacting atoms.
Table of Contents
- Q. What did John Dalton contribute to the understanding of the atom?
- Q. What contribution did John Dalton make to atomic theory quizlet?
- Q. What are the 4 main points of John Dalton’s atomic theory?
- Q. How many atoms are in our body?
- Q. What are humans made up of?
- Q. Is human body made of atoms?
- Q. What is an atomic number for?
- Q. Do atoms have life?
- Q. What is the most basic form of matter?
- Q. Who first gave the concept of atom?
- Q. What is the smallest particle on Earth?
- Q. Which of these is the smallest particle?
- Q. What particle is smaller than a quark?
Q. What contribution did John Dalton make to atomic theory quizlet?
John Dalton’s Atomic Theory included these statements. (1) All matter is composed of tiny, indivisible particles, called atoms, that cannot be destroyed or created. (2) Each element has atoms that are identical to each other in all of their properties.
Q. What are the 4 main points of John Dalton’s atomic theory?
Dalton’s Atomic Theory
- All matter is made of atoms. Atoms are indivisible and indestructible.
- All atoms of a given element are identical in mass and properties.
- Compounds are formed by a combination of two or more different kinds of atoms.
- A chemical reaction is a rearrangement of atoms.
Q. How many atoms are in our body?
Short Answer. There are approximately 7 x 1027 atoms in the average human body. This is the estimate for a 70 kg adult human male. Generally, a smaller person would contain fewer atoms; a larger person would contain more atoms.
Q. What are humans made up of?
Almost 99% of the mass of the human body is made up of six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. Only about 0.85% is composed of another five elements: potassium, sulfur, sodium, chlorine, and magnesium. All 11 are necessary for life.
Q. Is human body made of atoms?
The particles we’re made of About 99 percent of your body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. You also contain much smaller amounts of the other elements that are essential for life. Nuclei are around 100,000 times smaller than the atoms they’re housed in.
Q. What is an atomic number for?
The atomic number is the number of protons in the nucleus of an atom. The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
Q. Do atoms have life?
Since an atom has a finite number of protons and neutrons, it will generally emit particles until it gets to a point where its half-life is so long, it is effectively stable. It undergoes something known as “alpha decay,” and it’s half-life is over a billion times longer than the current estimated age of the universe.
Q. What is the most basic form of matter?
The basic unit of all matter is the atom. The atom is the smallest unit of matter that can’t be divided using any chemical means and the building block that has unique properties.
Q. Who first gave the concept of atom?
The ancient atomic theory was proposed in the 5th century bc by the Greek philosophers Leucippus and Democritus and was revived in the 1st century bc by the Roman philosopher and poet Lucretius.
Q. What is the smallest particle on Earth?
Quarks
Q. Which of these is the smallest particle?
atom
Q. What particle is smaller than a quark?
preons