Science Chapter 17 Test
Q. How do you combine two reactions?
You combine multiple reactions into a single equation by listing all the reactants on the left side of the equation and all the products on the right side of the equation. Simplification of the overall equation will eliminate chemical species that exist on both sides of the equation without change.
Table of Contents
- Q. How do you combine two reactions?
- Q. What is formed when two or more substances are combined answer?
- Q. Is smoke a compound True or false?
- Q. Is smoke a compound or solution?
- Q. Is smoke a compound or element?
- Q. What is a compound simple definition?
- Q. Is bleach a compound?
- Q. How important is compound?
- Q. What is true of a compound?
Q. What is formed when two or more substances are combined answer?
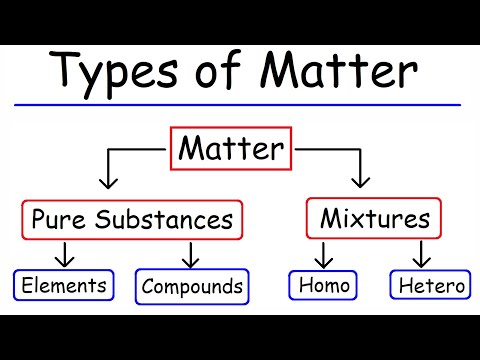
Two or more elements combined into one substance through a chemical reaction, such as water, form a chemical compound. A common example of a chemical substance is pure water; it always has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory.
| A | B |
|---|---|
| When two or more substances are combined so each substance can be separated by physical means, the result is a_________. | mixture |
| A __________ is NOT a mixture | compound |
| The scattering of light by colloids is called________. | The Tyndall Effect |
| _______ is NOT a homogeneous mixture | Permanent-Press Fabric |
Q. Is smoke a compound True or false?
Smoke is a compound. A substance can be separated into simpler parts through differences in physical properties. The law of conservation of mass states that the mass of all substances before a chemical change equals the mass of all the substances after the change. When a firecracker explodes, mass is lost.
Q. Is smoke a compound or solution?
Colloids are generally considered heterogeneous mixtures, but have some qualities of homogeneous mixtures as well. Smoke is a mixture of particles that are suspended in the air. Tap water is a mixture of water and other particles. Pure water or H2O is generally referred to as distilled water.
Q. Is smoke a compound or element?
Smoke is a mixture. Because in compound all constituents of a substance is in fixed ratio but it is not as in mixture. Hence, smoke is a mixture. Smoke is mixture..and in vide also a visible suspension of carbon or other particles in air, typically one emitted from a burning substance.
Q. What is a compound simple definition?
A compound is a material formed by chemically bonding two or more chemical elements. The type of bond keeping elements in a compound together may vary: covalent bonds and ionic bonds are two common types.
Q. Is bleach a compound?
What is bleach? Household bleach is actually a mixture of chemicals, Its main constituent is a solution of ~3-6% sodium hypochlorite (NaOCl), which is mixed with small amounts of sodium hydroxide, hydrogen peroxide, and calcium hypochlorite.
Q. How important is compound?
Compound interest makes a sum of money grow at a faster rate than simple interest, because in addition to earning returns on the money you invest, you also earn returns on those returns at the end of every compounding period, which could be daily, monthly, quarterly or annually.
Q. What is true of a compound?
Two things are true of all compounds: A compound always has the same elements in the same proportions. For example, carbon dioxide always has two atoms of oxygen for each atom of carbon, and water always has two atoms of hydrogen for each atom of oxygen. A compound always has the same composition throughout.