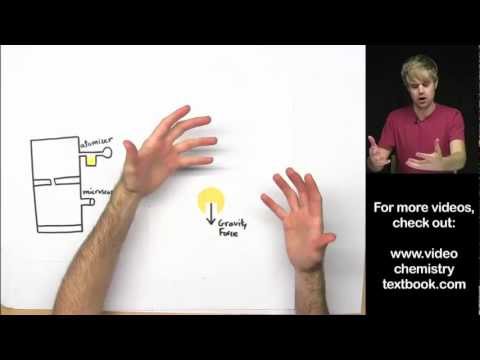
The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment took place in the Ryerson Physical Laboratory at the University of Chicago. Millikan received the Nobel Prize in Physics in 1923.
Q. What type of radiation did Millikan use to ionize the air in the chamber?
Using the microscope, he measured their terminal velocity, and by use of a formula, calculated the mass of each oil drop. Next, Millikan applied a charge to the falling drops by illuminating the bottom chamber with x-rays. This caused the air to become ionized, and electrons to attach themselves to the oil drops.
Table of Contents
- Q. What type of radiation did Millikan use to ionize the air in the chamber?
- Q. How did Millikan’s experiment allow determination of the electron’s mass?
- Q. Why in Millikan’s oil drop experiment the charge measured was always found to be of some discrete value and not any arbitrary value?
- Q. Which of the following charges can not be present on an oil drop in Millikan’s experiment?
- Q. Which of the following charge is not possible?
Q. How did Millikan’s experiment allow determination of the electron’s mass?
Millikan’s experiment determined the charge on the electron. Millikan suspended oil droplets between two electric plates and determined their charges. Oil droplets from a fine mist fell through a hole in the upper plate. From their terminal velocity, he could calculate the mass of each drop.
Q. Why in Millikan’s oil drop experiment the charge measured was always found to be of some discrete value and not any arbitrary value?
How does the electric flux, electric field enclosing a given charge vary when the area enclosed by the charge is doubled? Why in Millikan’s Oil Drop experiment, the charge measured was always found to be of some discrete value and not any arbitrary value? Ans: Because charge is always quantized ie., Q = n x e. 4.
Q. Which of the following charges can not be present on an oil drop in Millikan’s experiment?
Answer: The correct answer is Option a, c and d. Explanation: Millikan’s oil drop experiment is used to measure the charge of an electron. Before this experiment, the subatomic particles were not accepted.
Q. Which of the following charge is not possible?
The Possible values of electric charge are q = ± 1e; ± 2e; ± 3e… Charge less than the charge on an electron (i.e. e = 1.6 * 10-19 C) is not possible. Hence, C & D are not possible.