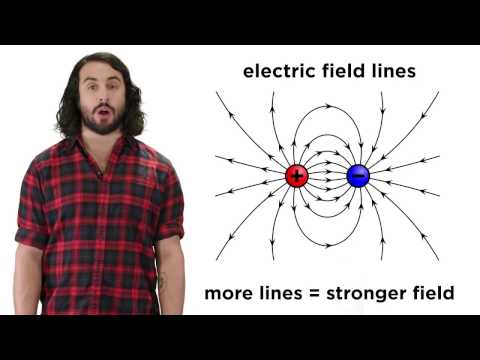
The pattern of the lines for the dipole indicates that the electric field is greatest in the region between and immediately surrounding the two charges, since the lines are closest together there.
Q. Where is an electric field strongest?
The field is strongest where the lines are most closely spaced. The electric field lines converge toward charge 1 and away from 2, which means charge 1 is negative and charge 2 is positive.
Table of Contents
- Q. Where is an electric field strongest?
- Q. Where would a test charge feel the strongest electric force?
- Q. What is the net electric field of a dipole?
- Q. What happens to dipole in electric field?
- Q. What is the charge of an electric dipole?
- Q. What is dipole moment example?
- Q. What is dipole moment explain with example?
- Q. What is SI unit of dipole moment?
- Q. What is electric dipole moment formula?
Q. Where would a test charge feel the strongest electric force?
The closer the test charge q is to the charge Q, the greater the force it will experience. Also, at points closer to the charge Q, the stronger is its electric field.
Q. What is the net electric field of a dipole?
The electric dipole moment associated with two equal charges of opposite polarity separated by a distance, d is defined as the vector quantity having a magnitude equal to the product of the charge and the distance between the charges and having a direction from the negative to the positive charge along the line between …
Q. What happens to dipole in electric field?
Electric dipole will experience a torque when dipole vector is not parallel to electric field direction. The torque τ = p × E = pESinθ. when p and E are parallel then angle θ between the two vectors is zero, hence there will not be any torque.
Q. What is the charge of an electric dipole?
The electric dipole moment vector p also points from the negative charge to the positive charge. An idealization of this two-charge system is the electrical point dipole consisting of two (infinite) charges only infinitesimally separated, but with a finite p.
Q. What is dipole moment example?
One of the most common examples is the water molecule, made up of one oxygen atom and two hydrogen atoms. The differences in electronegativity and lone electrons give oxygen a partial negative charge and each hydrogen a partial positive charge.
Q. What is dipole moment explain with example?
A dipole moment is simply the measure of net polarity in a molecule. Polar molecules exhibit a large difference in electrical charge (a positive end and a negative end), otherwise known as a dipole moment. For example, ammonia (NHsub3) is a polar molecule.
Q. What is SI unit of dipole moment?
The dipole moment of a dipole is defined as the product of one of the charges and the distance between them. p = q × 2a. The SI composite unit of electric dipole moment is the ampere second meter.
Q. What is electric dipole moment formula?
The formula for electric dipole moment for a pair of equal & opposite charges is p = qd, the magnitude of the charges multiplied by the distance between the two.