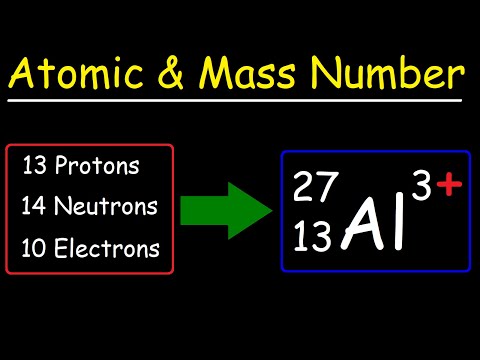
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Q. Which of the following elements has the lowest atomic mass?
The lightest chemical element is Hydrogen and the heaviest is Hassium. The unity for atomic mass is gram per mol.
Table of Contents
- Q. Which of the following elements has the lowest atomic mass?
- Q. Which element has the lowest atomic number?
- Q. What does the mass number tell you?
- Q. Which number is the atomic mass?
- Q. What 3 things does the atomic number tell you?
- Q. Why an atom has no overall charge?
- Q. Why do most things have no net charge?
- Q. How do you tell if it is an atom or ion?
- Q. How do you know if an ion is present?
- Q. How do you find an ion symbol?
- Q. Is a cation positive?
- Q. What is cation example?
- Q. Are cations positive or negative?
- Q. Why anode is negative?
- Q. Is anode negative or positive?
- Q. Is anode always positive?
- Q. Is anode acidic or basic?
- Q. Is potassium negative or positive?
- Q. What’s the electron configuration for K+?
Q. Which element has the lowest atomic number?
helium
Q. What does the mass number tell you?
The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. For example, the mass number of argon atoms and calcium atoms can both be 40.
Q. Which number is the atomic mass?
Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2+2=4, we know that the mass number of the helium atom is 4….Mass Number.
| Name | helium |
|---|---|
| Symbol | He |
| Atomic Number (Z) | 2 |
| Protons | 2 |
| Neutrons | 2 |
Q. What 3 things does the atomic number tell you?
The three main atomic particles are protons, neutrons and electrons. The atomic number of an atom identifies the number of protons in the atom. This is the defining characteristic of an element.
Q. Why an atom has no overall charge?
Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral.
Q. Why do most things have no net charge?
For an object to have no net change the protons and electrons would have to be equal. Protons and electrons like to be equal and matched up therefore most things have no net charge. They have the same charge at the end and repel each other.
Q. How do you tell if it is an atom or ion?
Atoms are neutral; they contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
Q. How do you know if an ion is present?
If an atom has the same number of protons and electrons, it is electronically neutral. However, if the total number of electrons does not equal the number of protons, the atom has a net electrical charge. Any atom or molecule with a net charge, either positive or negative, is known as an ion.
Q. How do you find an ion symbol?
When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or – (for negative ions or anions). Neutral atoms have a charge of zero, so no superscript is given.
Q. Is a cation positive?
What is a cation? A cation has more protons than electrons, consequently giving it a net positive charge.
Q. What is cation example?
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Some examples of cations are Calcium (Ca2+), Potassium (K+), hydrogen (H+).
Q. Are cations positive or negative?
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
Q. Why anode is negative?
Anode is negative in electrochemical cell because it has a negative potential with respect to the solution while anode is positive in electrolytic cell because it is connected to positive terminal of the battery.
Q. Is anode negative or positive?
In a battery or other source of direct current the anode is the negative terminal, but in a passive load it is the positive terminal. For example, in an electron tube electrons from the cathode travel across the tube toward the anode, and in an electroplating cell negative ions are deposited at the anode.
Q. Is anode always positive?
Anode and Cathode The battery anode is always negative and the cathode positive. This appears to violate the convention as the anode is the terminal into which current flows. A vacuum tube, diode or a battery on charge follows this order; however taking power away from a battery on discharge turns the anode negative.
Q. Is anode acidic or basic?
The anode is negative and the cathode is positive because the chemistry is stripping electrons off it faster than they can get replaced through the external circuit. In an electrolytic cell, where an external source of energy is driving a non-spontaneous chemistry, the resistance is in the cell.
Q. Is potassium negative or positive?
The important ions in the nervous system are sodium and potassium (both have 1 positive charge, +), calcium (has 2 positive charges, ++) and chloride (has a negative charge, -).
Q. What’s the electron configuration for K+?
[Ar] 4s¹