Sodium chloride
Q. What ways can sodium chloride conduct electricity?
Solid sodium chloride does not conduct electricity, because there are no electrons which are free to move. When it melts, sodium chloride undergoes electrolysis, which involves conduction of electricity because of the movement and discharge of the ions. In the process, sodium and chlorine are produced.
Table of Contents
- Q. What ways can sodium chloride conduct electricity?
- Q. Why sodium chloride conducts electricity when molten but not in solid state?
- Q. Which is responsible for electricity conduction of molten sodium chloride?
- Q. What is the melting point of NaCl?
- Q. Which is more ionic NaCl or KCl?
- Q. What is called Fajan’s rule?
- Q. Which is more ionic NaCl or mgcl2?
- Q. Is LiCl or KCl stronger?
- Q. Why LiCl is more covalent than KCl?
- Q. Why LiCl is more covalent than NaCl and KCl?
- Q. Is NaCl 100 Ionic?
- Q. Which is more soluble KCl or LiCl?
- Q. Which is more soluble in water NaCl or KCl?
- Q. Is NaCl or CaCl2 more soluble?
- Q. Why is KCl least soluble?
- Q. In which of the following NaCl is more soluble?
- Q. In which of the following KCl is more soluble?
- Q. Is potassium chloride KCl soluble or insoluble in water?
- Q. Which solvent would the solubility of KCl be greatest?
- Q. Which of the following solvents KCl is more soluble at 25 degrees Celsius?
- Q. What is the solubility of sodium nitrate at 45 C?
- Q. What is the solubility of KCl at 25 C *?
Q. Why sodium chloride conducts electricity when molten but not in solid state?
In an ionic compound, such as solid sodium chloride the free electrons are not present. Electrons are bound in bonds by strong electrostatic forces. So, sodium chloride does not conduct electricity in a solid-state. So, the reason for the conductivity of sodium chloride in the molten state is the presence of free ions.
Q. Which is responsible for electricity conduction of molten sodium chloride?
So a molten salt powers electricity. Hence, molten sodium chloride conducts electricity due to the presence of free ions. So, the correct answer is option D. Note- The flow of electricity in chemical compounds can be due to free electrons as well as free ions.
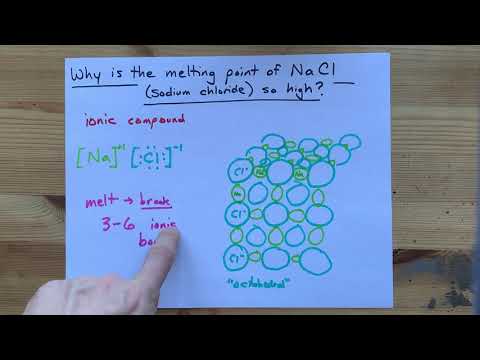
Q. What is the melting point of NaCl?
801 °C
Q. Which is more ionic NaCl or KCl?
KCl is more ionic because the ionic character increases as down the group while decreases along the period in the periodic table. Also, the bonding in KCl is more ionic than in NaCl. Electronegativity scales also support that NaCl is less ionic when compared to KCl.
Q. What is called Fajan’s rule?
From Wikipedia, the free encyclopedia. In inorganic chemistry, Fajans’ rules, formulated by Kazimierz Fajans in 1923, are used to predict whether a chemical bond will be covalent or ionic, and depend on the charge on the cation and the relative sizes of the cation and anion.
Q. Which is more ionic NaCl or mgcl2?
The covalent character is determined using Fajan’s rule. In the given compounds, the anion is the same and the cations are different. Therefore, MgCl2 is more covalent than NaCl. …
Q. Is LiCl or KCl stronger?
Because between LiCl & KCl cation size is decreasing in order Li is smaller than K and we know with decrease of size of cation increase the polarising power of cation. So polarishing power of Li is greater than K. So LiCl is more covalent than KCl.
Q. Why LiCl is more covalent than KCl?
In LiCl and KCl the anions are the same. From the periodic table, we can see that Li+ is smaller than K+.So in LiCl more polarisation occurs and makes it more covalent than KCl.
Q. Why LiCl is more covalent than NaCl and KCl?
a Due to smaller size and high poalrising power of Li+ ion LiCl is more covalent than KCl. b Small cation Li+ ion highly polarizes large anion I- LiI is more covalent and hence has lower melting point. c Due to pseudo inert gas configuration Cu+ is more polarizing than Na+ and hence CuCl is more Covalent NaCl.
Q. Is NaCl 100 Ionic?
The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl−.
Q. Which is more soluble KCl or LiCl?
Smaller the cation, greater is its polarizing power and hence larger is the covalent character. Thus LiCl is more covalent than KCl and hence KCl is more soluble in water because of decreased covalent character.
Q. Which is more soluble in water NaCl or KCl?
NaCl is more soluble in water than KCl. Solubility depends on the size of the cation as the anion is the same. The solubility of NaCl is 359 g/L at 200C and the solubility of KCl is 344 g/L at 200C.
Q. Is NaCl or CaCl2 more soluble?
For example, the solubility of CaCl2 at room temperature is listed on Wikipedia as 64.7 g/ 100 g water, whereas the solubility of NaCl is listed as 35.72 g/ 100 g water. At room temperature, the density of a saturated solution of CaCl2 is 1.435 g/ml and the density of a saturated solution of NaCl is 1.199 g/ml.
Q. Why is KCl least soluble?
For any compound to be soluble in water, It must satisfied the given below condition : Hydration energy of compound should be greater than its lattice energy. Hydration energy of potassium chloride is less than its lattice enthalpy so it is insoluble in water.
Q. In which of the following NaCl is more soluble?
NaCl is an ionic compound. So it’s solubility is maximum in another ionic compound. So water is the only ionic compound in the given options, so B is the correct answer.
Q. In which of the following KCl is more soluble?
Like dissolves like. Since KCl is an ionic compound, it will be more soluble in polar solvents which have a high dielectric constant. The solubility of KCl is relatively more in CH3OH(D=32), as both are polar molecules.
Q. Is potassium chloride KCl soluble or insoluble in water?
KCl – also known as sylvite – is freely soluble in water because of having electrolytic nature. In KCl, K stands for potassium and Cl stands for chlorine. Both of them when combined form the salt that is known as potassium chloride.
Q. Which solvent would the solubility of KCl be greatest?
In which solvent is each of the following more likely to be soluble? KCl soluble in water. Explanation: KCl is a ionic compound ( K+ + Cl–), in the solid state of it electrostatic force of attraction strongly binds the K+ and Cl– together.
Q. Which of the following solvents KCl is more soluble at 25 degrees Celsius?
Like dissolves like. Since KBr is a polar ionic compound, it will be soluble in a polar solvent. The solvent with a higher value of dielectric constant will provide maximum solubility. It will be most soluble in polar solvents, i.e. with the highest value of D.
Q. What is the solubility of sodium nitrate at 45 C?
Task 1: Producing a graph to represent solubility data
| Potassium nitrate (KNO3) | Sodium nitrate (NaNO3) | |
|---|---|---|
| Temp. °C | g / 100 g water | g / 100 g water |
| 30 | 45 | 96 |
| 40 | 63 | 105 |
| 50 | 84 | 114 |
Q. What is the solubility of KCl at 25 C *?
1 Answer. Truong-Son N. Once you look it up, you should be telling me that… I get 360 g KClkg water at 25∘C off of Wikipedia.