Q. Which element has the following ground state electron configuration 1s22s22p6?
element Silicon
Q. Which element has the following ground state electron configuration 1s 2 2s 2 2p 6 3s 2?
Magnesium
Table of Contents
- Q. Which element has the following ground state electron configuration 1s22s22p6?
- Q. Which element has the following ground state electron configuration 1s 2 2s 2 2p 6 3s 2?
- Q. Which ion has the electron configuration 1s22s22p63s23p6?
- Q. Which element has an electron configuration that ends in 4p3?
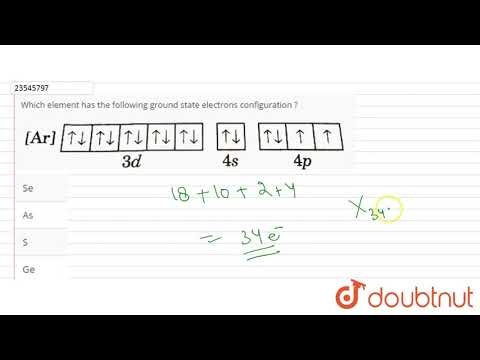
- Q. What element is AR 3d2?
- Q. What is the abbreviated ground state electron configuration for nitrogen?
- Q. What is the ground state electron configuration expected for cesium?
- Q. What is the electron configuration for tungsten?
- Q. How many electrons are in the 5d sublevel in the ground state configuration of tungsten?
- Q. How many electrons are in the 5s sublevel in the ground state configuration of silver?
- Q. Why is silver an exception to electron configuration?
- Q. Why is Ag+ more stable than Ag2+?
- Q. Which oxidation state of iron is most stable?
Q. Which ion has the electron configuration 1s22s22p63s23p6?
P3–: From Table 2.2, the electron configuration for an atom of phosphorus is 1s22s22p63s23p3. In order to become an ion with a minus three charge, it must acquire three electrons—in this case another three 3p. Thus, the electron configuration for a P3– ion is 1s22s22p63s23p6.
Q. Which element has an electron configuration that ends in 4p3?
[Ar] 4s2 3d10 4p3 is the electron configuration of a(n) atom. Which is the correct ground state electron configuration for silver? At maximum, an f-subshell can hold ____ electrons, d-shell can hold ____ and p-shell can hold ____ electrons. Which 2 elements have the same ground state electron configuration?
Q. What element is AR 3d2?
The inner shells of this species has the noble gas, Argon (Ar) configuration while its valence configuration is 3d².
Q. What is the abbreviated ground state electron configuration for nitrogen?
2s2.2p3
Q. What is the ground state electron configuration expected for cesium?
The ground state electron configuration of ground state gaseous neutral caesium is [Xe]. 6s1 and the term symbol is 2S1/2.
Q. What is the electron configuration for tungsten?
[Xe] 6s² 4f¹⁴ 5d⁴
Q. How many electrons are in the 5d sublevel in the ground state configuration of tungsten?
1 Answer. There are four unpaired electrons in the 5d sublevel (subshell).
Q. How many electrons are in the 5s sublevel in the ground state configuration of silver?
Silver atoms have 47 electrons and the shell structure is 2.8. 18.18. 1. The ground state electron configuration of ground state gaseous neutral silver is [Kr].
Q. Why is silver an exception to electron configuration?
Now, you have to be a little careful with silver because it is a transition metal, which implies that the occupied d-orbitals are actually lower in energy than the s-orbitals that belong to the highest energy level. The thing to remember here is that in silver’s case, the 4d orbitals will be completely filled.
Q. Why is Ag+ more stable than Ag2+?
Explanation: Ag+ is more stable than Ag2+ Cation due to the complete Ag+ configuration. An oxidizing agent always tends to accept the electrons. It is extremely volatile and therefore easily accepts electrons to achieve a more stable + 1 oxidation state.
Q. Which oxidation state of iron is most stable?
Iron is almost always encountered in the oxidation states 0 (as in the metal), +2, or +3. Iron(III) is usually the most stable form in air, as illustrated by the pervasiveness of rust, an insoluble iron(III)-containing material.