Q. Which is the correct formula for an annulene?
Annulene. Annulenes are completely conjugated monocyclic hydrocarbons. They have the general formula C n H n (when n is an even number) or C n H n+1 (when n is an odd number). The IUPAC naming conventions are that annulenes with 7 or more carbon atoms are named as [ n ]annulene, where n is the number of carbon atoms in their ring,…
Q. What is the bond length of benzene annulene?
The bond lengths of [18]annulene are around 139 to 141 pm, indicating delocalization and a bond order between 1 and 2. In general, carbon-carbon single bonds and double bonds are 154 pm and 134 pm, respectively, while benzene, with a bond order of 1.5, the bond-length is 140 pm.
Q. Are there any annulenes which contain conjugated double bonds?
Yes, these are the annulenes which are monocyclic, containing conjugated double bonds and prefixed with their ring size. As annulenes are monocyclic rings with conjugated double bonds, the molecular formula linearly increases with ring size with a little difference with odd number or even number.
Q. Which is the most stable isomer of annulene?
The usual isomer that [18]annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis, trans, trans, cis, trans, trans, cis, trans, trans configuration. It is reported to be a red-brown crystalline solid.
Q. What is the formula for a dehydrobenzo annulene?
Structure and AFM image of dehydrobenzo[12]annulene. Annulenes are completely conjugated monocyclic hydrocarbons. They have the general formula CnHn (when n is an even number) or CnHn+1 (when n is an odd number).
Q. How is the planarity of an annulene maintained?
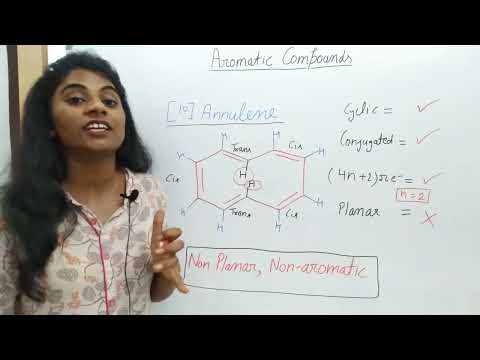
[14]annulene was first synthesized. It has two isomers as shown above which are in equilibrium. This molecule has 4n+2 π (n=3) electrons. In this case, as the size of the ring is large (14 carbon atoms), the internal H ‘s are not too close to each other to cause steric strain. Thus, this molecule can maintain its planarity and be aromatic.
Q. Which is the only annulene with antiaromaticity?
Annulenes may be aromatic (benzene, [6]annulene and [18]annulene), non-aromatic ( [8] and [10]annulene), or anti-aromatic (cyclobutadiene, [4]annulene). Cyclobutadiene is the only annulene with considerable antiaromaticity, since planarity is unavoidable.