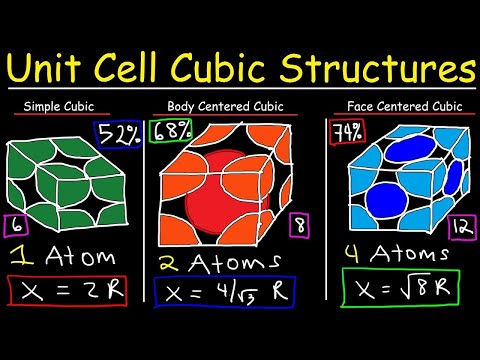
Both ccp and hcp are highly efficient lattice; in terms of packing. The packing efficiency of both types of close packed structure is 74%, i.e. 74% of the space in hcp and ccp is filled. The packing efficiency of simple cubic lattice is 52.4%. And the packing efficiency of body centered cubic lattice (bcc) is 68%.
Q. What is the formula of packing efficiency?
Packing efficiency can be written as below, Packing efficiency = Volume occupied by 6 spheres ×100 / Total volume of unit cells. Examples are Magnesium, Titanium, Beryllium etc. In body-centered cubic structures, the three atoms are arranged diagonally.
Table of Contents
- Q. What is the formula of packing efficiency?
- Q. Which crystal structure has the greatest packing efficiency?
- Q. What type of crystal lattice has the highest packing efficiency?
- Q. What is the highest packing efficiency?
- Q. Which of the following is the most effective packing arrangement?
- Q. Which metal has highest packing efficiency?
- Q. Which of the following packing is 74%?
- Q. What does packing efficiency of a crystal structure tell us?
- Q. Which element has lowest packing efficiency?
- Q. Which of the following three types of packing is most efficient?
- Q. Which packing fraction is best?
- Q. What is the formula of packing fraction?
- Q. What is maximum packing fraction?
- Q. What is the relation between A and R for BCC?
- Q. What is the atomic radius of a bcc crystal structure?
- Q. What is the atomic radius of a bcc crystal structure formula?
- Q. What is the atomic radius of a bcc crystal structure Mcq?
- Q. Which of the following is point defect in Crystal?
- Q. Which of the combination of crystal structure and their coordination number is correct?
- Q. How many types of crystal systems are there in total?
Q. Which crystal structure has the greatest packing efficiency?
Crystal Structure: Closest Packing
- The most efficient conformation atomic spheres can take within a unit cell is known as the closest packing configuration.
- Densely packed atomic spheres exist in two modes: hexagonal closest packing (HCP) and cubic closest packing (CCP).
Q. What type of crystal lattice has the highest packing efficiency?
Hexagonal
Q. What is the highest packing efficiency?
Hexagonal close-packed lattice has the highest packing efficiency of 74%. The packing efficiencies of simple cubic and body-centred cubic lattices are 52.4% and 68% respectively. Was this answer helpful?
Q. Which of the following is the most effective packing arrangement?
– The packing arrangement ABC ABC type in 3 D is equivalent to cubic closed packing (ccp) or face centred cubic (fcc) lattice. – The efficiency of fcc is the highest among all the arrangements, i.e., 74%. – Hence, ABC ABC type arrangement in 3 D is the most efficient.
Q. Which metal has highest packing efficiency?
Among the given metals Al occupies hexagonal close-packed (hcp) is having 0.74 packing efficiency and other metals form body-centered cubic (bcc) which has 0.68 packing efficiency.
Q. Which of the following packing is 74%?
(i) In a simple cubic lattice the atoms are located only on the corners of the cube. Hexagonal close−packed lattice has the highest packing efficiency of 74%.
Q. What does packing efficiency of a crystal structure tell us?
The packing efficiency of a crystal structure tells us how much of the available space is being occupied by atoms. It is usually represented by a percentage or volume fraction. Count how many atoms there are per unit cell. Calculate the volume of a single atom and multiply by the number of atoms in the unit cell.
Q. Which element has lowest packing efficiency?
Simple cubic unit cell has least packing efficiency that is 52.4%.
Q. Which of the following three types of packing is most efficient?
Out of the three types of packing, face-centered cubic (or ccp or hcp) lattice makes the most efficient use of space while simple cubic lattice makes the least efficient use of space.
Q. Which packing fraction is best?
The highest packing fraction possible is 74.04 % and this is for the FCC lattice. The same value of packing fraction is for the HCP structure as well, which only differs from FCC in that the location of the third layer is different (ababab), but the number of atoms in a given volume is identical in FCC and HCP.
Q. What is the formula of packing fraction?
Hint: In the structure of diamond cubic crystal the unit cell present is fcc unit cell. The relation between r and a for diamond is r=√3a8. Complete answer: Packing fraction (P.F), is calculated by volume occupied by the number of spheres in the unit cell divided by volume of a unit cell.
Q. What is maximum packing fraction?
The maximum packing fraction is not a well-defined parameter as it depends on the spatial distribution of the elements, which may depend on the flow history. For uniform spheres it is around 55% if the particles are simply poured in a container and can be increased to 64% by a slight and long vibration.
Q. What is the relation between A and R for BCC?
The relation between edge length (a) and radius of atom (r) for BCC lattice is √3a=4r .
Q. What is the atomic radius of a bcc crystal structure?
Chromium has BCC structure. Its atomic radius is 0.1249 nm.
Q. What is the atomic radius of a bcc crystal structure formula?
Packing Density When the lattice points are inflated gradually, at some point they start to touch each other along the diagonals of the cube. One can now interpret the atoms as close packed spheres with a radius defined geometrically by 4r=√3a 4 r = 3 a ⇔r=√34a ⇔ r = 3 4 a .
Q. What is the atomic radius of a bcc crystal structure Mcq?
What is the atomic radius of a BCC crystal structure? Explanation: Atomic radius is defined as half the distance between the centers of two neighboring atoms. The atomic radius of a simple cube and HCP is a/2 respectively, whereas it is a√2/4 and a√3/4 for FCC and BCC respectively. 17.
Q. Which of the following is point defect in Crystal?
1. Which of the following is a point defect in crystals? Explanation: Crystal defects are classified as point defects, line defects, and boundary defects. Point defects include vacancies, impurities, interstitialcies, and electronic defects.
Q. Which of the combination of crystal structure and their coordination number is correct?
Which of the combinations of crystal structure and their coordination number is (are) correct ? Option (B) is wrong because fcc has 12 coordination no.
Q. How many types of crystal systems are there in total?
seven crystal systems