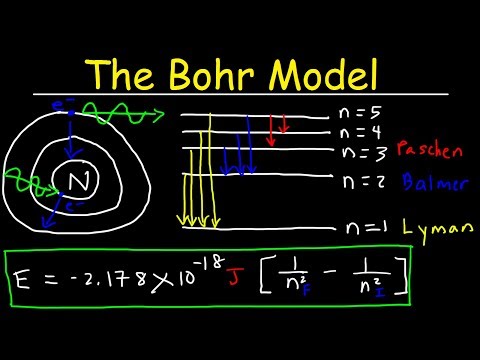
A photon with an energy of 10.2 eV has a wavelength of 1.21 x 10-7 m, in the ultraviolet part of the spectrum. So when an electron wants to jump from n = 1 to n = 2, it must absorb a photon of ultraviolet light.
Q. Do electrons always fall back to ground state?
Electrons do not stay in excited states for very long – they soon return to their ground states, emitting a photon with the same energy as the one that was absorbed.
Table of Contents
- Q. Do electrons always fall back to ground state?
- Q. Why do electrons stay in ground state?
- Q. Is the energy difference between n 3 and n 4 is larger than the energy difference between N 1 and N 2?
- Q. What are the brightest wavelengths of light in hydrogen?
- Q. What colors of light are absorbed by helium?
- Q. Do all colors of light travel at the same speed?
- Q. Which color refracts the most?
Q. Why do electrons stay in ground state?
Let me explain further, as electron gets closer to an atom the electrostatic & other quantum mechanical forces between the electron starts pulling it, and as electron gets closer the electron is essentially more confined and since Heisenberg’s uncertainty principle governs that the electrons momentum has to become …
Q. Is the energy difference between n 3 and n 4 is larger than the energy difference between N 1 and N 2?
The energy difference between the n = 4 and n = 3 orbitals is larger than the energy difference between the n = 3 and n = 2 orbitals.
Q. What are the brightest wavelengths of light in hydrogen?
An element produces bright and dark lines with the same wavelengths. For example, hydrogen has three prominent lines with wavelengths of 434 nm, 486 nm, and 656 nm; these appear dark if the hydrogen is absorbing light, and bright if it is emitting light, but the same three wavelengths are seen in either case.
Q. What colors of light are absorbed by helium?
What colors of light are absorbed by helium gas? primarily the yellow,blue,blue-green and red but also violet and blue are somewhat absorbed. In 1913, Niels Bohr proposed that the unique spectral lines created by different elements were related to the way electrons were arranged around the nucleus.
Q. Do all colors of light travel at the same speed?
The different colors of light all travel at roughly the same speed in air. In glass, however, this is not true. Blue light gets slowed down by glass more than red light. As a result of their different speeds in the glass, the red and blue light get bent at different angles when they go into the prism.
Q. Which color refracts the most?
violet light