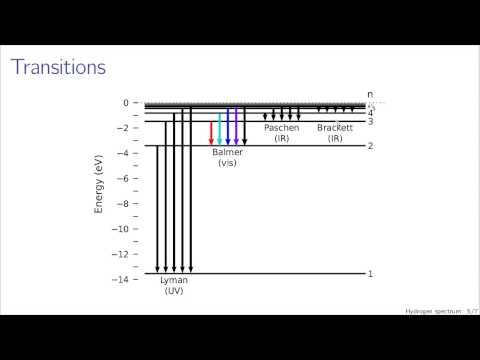
Helium is a bit more complicated, and the elements below get increasingly complicated. Hydrogen spectrum is simple because there is only one electron in a hydrogen atom. A helium atom has two electrons, so there are more possibilities for the excited states.
Q. Why do helium and hydrogen have different emission spectra?
There are different interactions present in helium atom such as electron-electron repulsion and nuclei-electron repulsion. So, due to the presence of different energy levels, there is emission of different wavelengths in helium atom. Therefore, hydrogen emission wavelength is different from helium emission wavelength.
Table of Contents
- Q. Why do helium and hydrogen have different emission spectra?
- Q. Does hydrogen or helium have more energy?
- Q. Does each element have a unique emission spectrum?
- Q. What is the difference between an absorption and an emission spectrum?
- Q. What is difference between spectra and spectrum?
- Q. What is normal spectrum?
- Q. What are the types of spectrum?
- Q. What is an example of a spectrum?
- Q. What is spectrum in data communication?
- Q. What do you mean by order of spectrum?
- Q. What is the absorption spectrum used for?
- Q. What’s the purpose of an absorption spectrum?
Q. Does hydrogen or helium have more energy?
The short answer is that it’s because helium has more electrons than hydrogen. In hydrogen, it’s common for more than one state to have the same energy. That’s called “degeneracy.” The extra electron in helium breaks these degeneracies. Also, there are more different ways for the electrons to fill orbitals.
Q. Does each element have a unique emission spectrum?
The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify unknown elements.
Q. What is the difference between an absorption and an emission spectrum?
The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum, whereas an absorption spectrum has dark-coloured lines in the spectrum.
Q. What is difference between spectra and spectrum?
A spectrum (plural spectra or spectrums) is a condition that is not limited to a specific set of values but can vary, without steps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors in visible light after passing through a prism.
Q. What is normal spectrum?
Normal spectrum a representation of a spectrum arranged upon conventional plan adopted as standard, especially a spectrum in which the colors are spaced proportionally to their wave lengths, as when formed by a diffraction grating.
Q. What are the types of spectrum?
Types of spectra – Emission spectra, Line spectrum, Band Spectrum, Absorption Spectra
- (i) Emission spectra.
- Continuous spectrum.
- Line spectrum.
- Band Spectrum.
- (ii) Absorption Spectra.
- 1 Fraunhofer lines.
- Fluorescence.
- Phosphorescence.
Q. What is an example of a spectrum?
A natural example of a spectrum is a rainbow. The word spectrum was first used by scientists studying optics. They used the word to describe the rainbow of colors in visible light when separated using a prism. The spectrum seen when light passes through a prism is an example of the dispersion of light.
Q. What is spectrum in data communication?
Spectrum relates to the radio frequencies allocated to the mobile industry and other sectors for communication over the airwaves. Additional frequencies, including both coverage and capacity bands, means mobile operators can connect more people and offer faster speeds.
Q. What do you mean by order of spectrum?
The light flux in the family of deviated rays that emerge after diffraction at the grating exhibit pronounced maxima along well-defined and enumerable directions on each side of the undeviated beam. …
Q. What is the absorption spectrum used for?
Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications.
Q. What’s the purpose of an absorption spectrum?
Absorption spectroscopy works as an analytical chemistry tool that can determine if a particular substance is present in a sample and often also quantify how much of the substance is present.