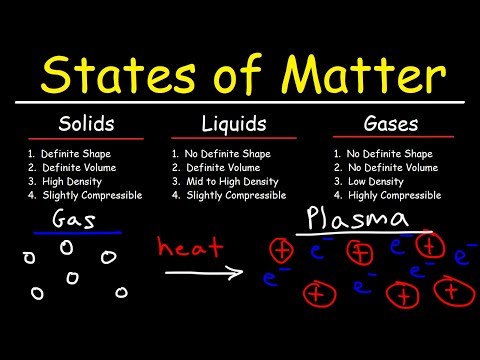
Molecules in a liquid have more energy than molecules in a solid. And if you heat it up even more, the molecules will speed up so much that they won’t be stuck together at all. The molecules in the gas have the most energy.
Q. Which phase has the highest potential energy?
solid
Table of Contents
- Q. Which phase has the highest potential energy?
- Q. Which phase has the highest kinetic energy Why?
- Q. Can you stop particles from moving?
- Q. What is the lifetime of plasma matter?
- Q. Where is plasma found naturally?
- Q. Why is it called plasma?
- Q. Is blood plasma and plasma the same thing?
- Q. What is the difference between plasm and plasma?
- Q. How can a gas turn into plasma?
- Q. Can a liquid turn into plasma?
- Q. What are the 7 types of phase changes?
- Q. What are six common phase changes?
- Q. What are phase changes called?
Q. Which phase has the highest kinetic energy Why?
Energy and State of Matter A pure substance in the gaseous state contains more energy than in the liquid state, which in turn contains more energy than in the solid state. Particles has the highest kinetic energy when they are in the gaseous state. Kinetic energy is related to heat (also called thermal energy).
Q. Can you stop particles from moving?
Absolute zero is the temperature at which the particles of matter (molecules and atoms) are at their lowest energy points. Some might think that at absolute zero particles lose all energy and stop moving. Therefore, a particle cannot be completely stopped because then its exact position and momentum would be known.
Q. What is the lifetime of plasma matter?
Ranges of plasma parameters
| Typical ranges of plasma parameters: orders of magnitude (OOM) | |
|---|---|
| Lifetime in seconds | 10−12 s (laser-produced plasma) to 107 s (fluorescent lights) (~19 OOM) |
| Density in particles per cubic metre | 107 m-3 to 1032 m-3 (inertial confinement plasma) |
Q. Where is plasma found naturally?
Because it consists of charged particles, plasma can conduct electricity and respond to a magnetic field. The sun and other stars consist of plasma. Plasma is also found naturally in lightning and the northern and southern lights. Human-made plasma is found in fluorescent lights, plasma TV screens, and plasma spheres.
Q. Why is it called plasma?
The word “plasma,” derived from the ancient Greek “to mold,” had been in use in medicine and biology for some decades when American chemist and physicist Irving Langmuir (1881-1957) began experimenting on electrical discharges in gas at the General Electric Research and Development Center in upstate New York.
Q. Is blood plasma and plasma the same thing?
Blood is the main bodily fluid and responsible for transporting important nutrients, oxygen, carbon dioxide and waste products to and away from the cells. Plasma is the yellow liquid component of blood and constitutes 55% of the total blood volume. Plasma is liquid component of blood.
Q. What is the difference between plasm and plasma?
As nouns the difference between plasma and plasm is that plasma is (physics) a state of matter consisting of partially ionized gas while plasm is a mold or matrix in which anything is cast or formed to a particular shape.
Q. How can a gas turn into plasma?
Just as a liquid will boil, changing into a gas when energy is added, heating a gas will form a plasma – a soup of positively charged particles (ions) and negatively charged particles (electrons).
Q. Can a liquid turn into plasma?
Summary: Researchers have found a way to turn a liquid metal into a plasma and to observe the temperature where a liquid under high-density conditions crosses over to a plasma state. Most laypersons are familiar with the three states of matter as solids, liquids, and gases. But there are other forms that exist.
Q. What are the 7 types of phase changes?
Phase Change: Evaporation, Condensation, Freezing, Melting, Sublimation & Deposition.
Q. What are six common phase changes?
Melting, freezing, vaporization, condensation, sublimation, and deposition are six common phase changes.
Q. What are phase changes called?
Phase Changes
| Phase Change | Name | Intermolecular Forces Increase or Decrease? |
|---|---|---|
| solid liquid | melting or fusion | increase decrease |
| liquid gas | vaporization or evaporation | increase decrease |
| gas solid | deposition | increase decrease |
| gas liquid | condensation | increase decrease |