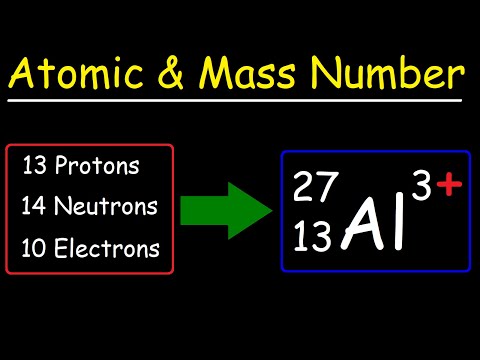
Q. Which term represents the sum of the atomic masses of the atoms in a molecule atomic number mass number Formula Mass percent composition by mass?
Isotopic masses
Q. What term refers to the sum of the atomic masses of all the atoms in a molecule?
The sum of mass of all the atoms in a molecule is known as molecular mass.
Table of Contents
- Q. Which term represents the sum of the atomic masses of the atoms in a molecule atomic number mass number Formula Mass percent composition by mass?
- Q. What term refers to the sum of the atomic masses of all the atoms in a molecule?
- Q. Which term represents the sum?
- Q. When referring to the sum of the atomic masses of the atoms in a chemical formula the correct term is?
- Q. What is the sum of the atomic masses of all the atoms in a formula for a compound?
- Q. What is the SI unit of mass?
- Q. What is the difference between weight and mass?
- Q. What is the symbol for mass?
- Q. What is the definition of mass and weight?
- Q. How does mass affect weight?
- Q. What did Galileo experiment prove?
- Q. Did Galileo know about gravity?
Q. Which term represents the sum?
The symbol Σ (sigma) is generally used to denote a sum of multiple terms. This symbol is generally accompanied by an index that varies to encompass all terms that must be considered in the sum. For example, the sum of first whole numbers can be represented in the following manner: 1 2 3 ⋯.
Q. When referring to the sum of the atomic masses of the atoms in a chemical formula the correct term is?
The molecular weight of a molecule is the sum of the atomic weights of its component atoms. If a substance has molecular weight M, then M grams of the substance is termed one mole. The number of molecules in one mole is the same for all substances; this number is known as Avogadro’s number (6.022140857 × 1023).
Q. What is the sum of the atomic masses of all the atoms in a formula for a compound?
formula mass
Q. What is the SI unit of mass?
The SI unit of mass is the kilogram (kg). In science and technology, the weight of a body in a particular reference frame is defined as the force that gives the body an acceleration equal to the local acceleration of free fall in that reference frame.
Q. What is the difference between weight and mass?
In scientific contexts, mass is the amount of “matter” in an object (though “matter” may be difficult to define), whereas weight is the force exerted on an object by gravity.
Q. What is the symbol for mass?
kg
Q. What is the definition of mass and weight?
The mass is essentially “how much stuff” is in an object. Weight: There is a gravitational interaction between objects that have mass. If you consider an object interacting with the Earth, this force is called the weight. The unit for weight is the Newton (same as for any other force).
Q. How does mass affect weight?
As your body grows, you will have more mass, which also means you will weigh more. That’s because when you’re on the earth, the amount of gravity that pulls on you stays the same. So when your mass changes, so does your weight!
Q. What did Galileo experiment prove?
According to the story, Galileo discovered through this experiment that the objects fell with the same acceleration, proving his prediction true, while at the same time disproving Aristotle’s theory of gravity (which states that objects fall at speed proportional to their mass).
Q. Did Galileo know about gravity?
In 1589, Galileo conducted experiments with gravity, such as dropping balls from the Leaning Tower of Pisa; he discovered that they hit the ground at the same time despite having different weights.