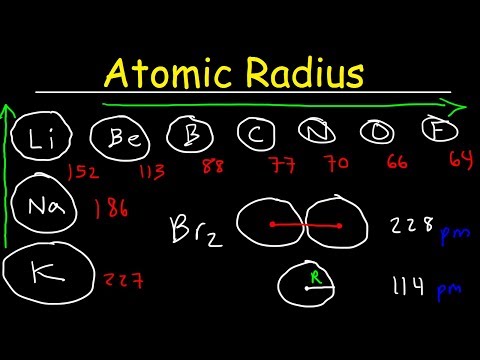
Q. Which trend is observed as the first four elements in group 17 are considered in order of increasing atomic numbers?
ANSWER: The melting and boiling points increase in order of increasing atomic number.
Q. Which general trend is found in period three has elements are considered in order of increasing atomic number?
Period 3 on the Periodic Table are considered in (3) Their metallic properties increase and their order of increasing atomic number? atomic radii decrease. (1) The atomic radius decreases, and the first Their metallic properties increase and their ionization energy generally increases.
Table of Contents
- Q. Which trend is observed as the first four elements in group 17 are considered in order of increasing atomic numbers?
- Q. Which general trend is found in period three has elements are considered in order of increasing atomic number?
- Q. Which element in Group 17 is most reactive?
- Q. Does ionic radius increase down Group 17?
- Q. Does ionic radius increase down a group?
- Q. Which element of group 17 exists as a solid at 25?
- Q. How can I memorize faster?
- Q. How do you teach the periodic table to students?
- Q. How do you remember the elements in group 16?
- Q. What is the most reactive element in Group 16?
- Q. Why are Group 16 called Chalcogens?
- Q. How do you remember elements?
- Q. What are the 20 element in chemistry?
- Q. What are the 20 elements and their symbols?
Q. Which element in Group 17 is most reactive?
fluorine
Q. Does ionic radius increase down Group 17?
ionic radii of group 17 anions increases down the group.
Q. Does ionic radius increase down a group?
As you move down a column or group, the ionic radius increases. Ionic radius decreases moving from left to right across a row or period. More protons are added, but the outer valence shell remains the same, so the positively charged nucleus draws in the electrons more tightly.
Q. Which element of group 17 exists as a solid at 25?
The melting point will higher than that of iodine (114°C) which is greater than room temperature (taken as 25°C) so astatine will be a solid at room temperature and pressure.
Q. How can I memorize faster?
How to Memorize More and Faster Than Other People
- Prepare.
- Record What You’re Memorizing.
- Write Everything Down.
- Section Your Notes.
- Use the Memory Palace Technique.
- Apply Repetition to Cumulative Memorization.
- Teach It to Someone.
- Listen to the Recordings Continuously.
Q. How do you teach the periodic table to students?
3 Free Lesson Ideas for Teaching the Periodic Table
- Interactive starter activity. Give each student a piece of card labelled with an element from group 1-7.
- Creating Element songs. Create a knowledge bank on the board with key words and facts about elements in the periodic table.
- Treasure Hunt.
Q. How do you remember the elements in group 16?
Group 16 is known as the group of Chalcogens or Oxygen group. It includes Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and the radioactive element Polonium (Po). Mnemonic for Group 16: Oh! Style Se Tel Polish.
Q. What is the most reactive element in Group 16?
Oxygen
Q. Why are Group 16 called Chalcogens?
Chalcogens means ore forming, as most of the ores in the earth crust are either oxides or sulphides, group 16 elements are called chalcogens. for example: Oxygen is the most abundant of all the elements on earth.
Q. How do you remember elements?
Memorization Strategies
- Break down the table into sections.
- Spread out the memorization process.
- Learn the elements in a song.
- Make nonsense words made from element symbols.
- Use color to learn element groups.
- Use a mnemonic device to help remember the order of the elements.
Q. What are the 20 element in chemistry?
The elements of the periodic table sorted by atomic number
| Atomic number | Name chemical element | Symbol |
|---|---|---|
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |
| 21 | Scandium | Sc |
Q. What are the 20 elements and their symbols?
First 20 Elements
| Atomic Number | Element | Symbol |
|---|---|---|
| 17 | Chlorine | Cl |
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |