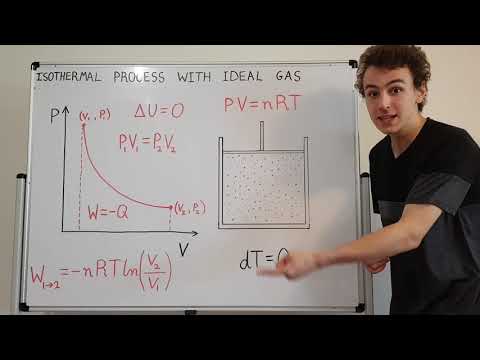
Solution : An isothermal process takes place at constant temperature, must be carried out in a vessel with conducting wall so that heat generated should go out at once.
Q. When an ideal gas is compressed isothermally what happens?
We know that when we compress a gas isothermally, its temperature is constant and volume of the gas decreases. Now let us consider the ideal gas equation. It is given as, PV=nRT, were ‘T’ is the temperature, ‘R’ is the ideal gas constant, ‘n’ is the number of moles, ‘V’ is the volume and ‘P’ is the pressure.
Table of Contents
- Q. When an ideal gas is compressed isothermally what happens?
- Q. Can an ideal gas be compressed?
- Q. When heat is given to a gas in an isothermal change the result will be?
- Q. Which is true of an adiabatic process?
- Q. Which process is reversible?
- Q. What is difference between reversible and irreversible process?
- Q. What does irreversible change mean?
- Q. What are some irreversible changes?
Q. Can an ideal gas be compressed?
In an ideal gas there is no temperature change upon compression or expansion.
Q. When heat is given to a gas in an isothermal change the result will be?
When heat is given to a gas in an isothermal change, the result will be External work done and also rise in temp.
Q. Which is true of an adiabatic process?
During an adiabatic process, there is no heat that flows in or out of the system. That means that Q=0. This means that any change in internal energy must come from work being done on or by the system.
Q. Which process is reversible?
Examples of Reversible Process slow adiabatic compression or expansion of gases. electrolysis (with no resistance in the electrolyte) the frictionless motion of solids. slow isothermal compression or expansion of gases.
Q. What is difference between reversible and irreversible process?
The basic difference between reversible and irreversible processes is that in the reversible process the system remains in thermodynamic equilibrium, while in the irreversible process the system does not remain in thermodynamic equilibrium.
Q. What does irreversible change mean?
A change is called irreversible if it cannot be changed back again. In an irreversible change, new materials are always formed. Sometimes these new materials are useful to us.
Q. What are some irreversible changes?
Irreversible changes are permanent changes that cannot be undone. Cooking, baking, frying, burning, mixing, rusting, heating are examples of irreversible changes.