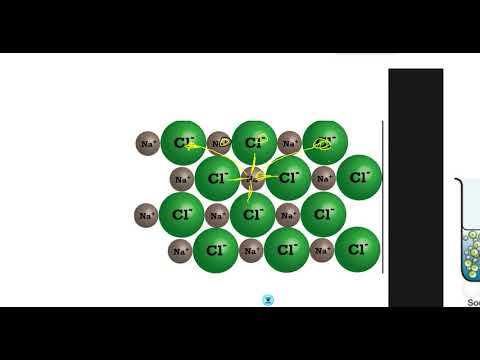
Q. Why can solutions of ionic compounds conduct electricity?
Aqueous solution of ionic compounds is able to conduct electricity because in aqueous solution, the strong force of attraction between ions is vanished. Their constituent ions get separated and become free to move across the solution.
Q. Why are ionic compounds conductive in water?
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely charged electrode.
Table of Contents
- Q. Why can solutions of ionic compounds conduct electricity?
- Q. Why are ionic compounds conductive in water?
- Q. When ionic compounds dissolve in water the solution will conduct electricity?
- Q. What type of solution can conduct an electrical current?
- Q. Which metal is best conductor?
- Q. Is oil a good conductor of electricity?
- Q. Is c6h1206 a good conductor of electricity?
- Q. Do salts conduct electricity?
- Q. Which salt is most conductive?
- Q. Can playdough conduct electricity?
- Q. Is playdough a good insulator?
- Q. How do you make electric playdough?
- Q. Is Clay a conductor of electricity?
- Q. Is sand an electrical conductor?
- Q. What is a good insulator?
- Q. Is tungsten a good conductor of electricity?
- Q. Which metal is bad conductor of electricity?
- Q. Why is tungsten used in an electric bulb?
- Q. What Tungsten is used in electric bulb?
- Q. Which gas is used in electric bulbs?
- Q. What is the resistivity of tungsten?
- Q. What material has the highest electrical resistance?
- Q. What has the highest resistivity?
- Q. What is the resistance of a 20.0 m long piece of 12 gauge?
- Q. What material has lowest electrical resistance?
- Q. What is the resistance of steel?
Q. When ionic compounds dissolve in water the solution will conduct electricity?
The capability of water to pass electricity current is called the electrical conductivity. It is considered as one of the physical properties of solutions. When ionic compounds dissolve in water they dissociate into ions.
Q. What type of solution can conduct an electrical current?
Electrolyte Solutions
Q. Which metal is best conductor?
What Metal is the Best Conductor of Electricity?
- Silver. The best conductor of electricity is pure silver, but to no surprise, it is not one of the most commonly used metals to conduct electricity.
- Copper. One of the most commonly used metals to conduct electricity is copper.
- Aluminum.
Q. Is oil a good conductor of electricity?
Oil is an insulator and is a bad conductor of electricity. Complete step by step answer: The oil by nature does not conduct electricity. The conductivity is dependent on several factors like base oil, water content, other additives and polarity.
Q. Is c6h1206 a good conductor of electricity?
Let’s now go over the compounds listed in answer choices: C3H7OH is a covalent compound (all the elements are nonmetals) and does not conduct electricity, C6H12O6 similarly is a covalent compound because it consists of all nonmetals. Both of these compounds can conduct electricity.
Q. Do salts conduct electricity?
Conducts Electricity For example, solid sodium chloride (NaCl, or table salt) does not conduct electricity; it is an insulator. Molten salts conduct electricity the same way they do when they are dissolved in water; some of the salt molecules are dissociated into ions, which allows the ions to conduct electricity.
Q. Which salt is most conductive?
These ions can move and carry a current effectively. The higher the concentration of ions, the greater the conductivity. Table salt, or sodium chloride, is an example of a compound with strong conductivity.
Q. Can playdough conduct electricity?
Why does play dough conduct electricity? Play-dough contains salt dissolved in water, which conducts electricity. The insulating dough contains sugar which does not conduct electricity.
Q. Is playdough a good insulator?
Salty water conducts electricity, which makes ordinary play dough a reasonably good conductor. Replacing the salt in play dough with sugar produces dough that conducts so poorly at low voltages that it functions like a non-conducting insulator.
Q. How do you make electric playdough?
Electric Play Dough Instructions
- Mix ingredients. Mix flour, salt, cream of tartar, and oil in a large mixing bowl.
- Add water. Slowly add the hot water.
- Knead it. Once it’s cool enough to handle, remove the dough from the bowl and begin kneading it.
- Electrify it!
- Add light.
- Test your dough.
Q. Is Clay a conductor of electricity?
The clay conductivity contributes effectively to the process of electric-current conduction, particularly when the medium is saturated with fresh water. In the present study, the conductivity of clays was investigated in relation to the formation resistivity factor and specific surface area.
Q. Is sand an electrical conductor?
Is it a good conductor of electricity? Sand is a terrible conductor of electricity. That’s why if it is struck by lightning, it heats to extremes and melts into a blob. Things don’t have to be very conductive to conduct from sources of billions to trillions of volts.
Q. What is a good insulator?
Plastic, rubber, wood, and ceramics are good insulators. These are often used to make kitchen utensils, such as saucepan handles, to stop heat from flowing up to burn the cook’s hand. Plastic coating is also used to cover most electrical wires in appliances. Air is also a good insulator of heat.
Q. Is tungsten a good conductor of electricity?
Tungsten is a poor conductor of electricity even though it is a metal. But at high temperatures, it will conduct electricity. As discussed above, it has a high melting point. Therefore, high temperature doesn’t have any bad effect on Tungsten.
Q. Which metal is bad conductor of electricity?
Tungsten
Q. Why is tungsten used in an electric bulb?
– It is solid at room temperature and possesses the highest melting point and lowest vapour pressure among all the metals. – It also has the highest tensile strength known. This property of tungsten makes it readily available for incandescent and fluorescent light bulb filaments.
Q. What Tungsten is used in electric bulb?
Tungsten is used in electric bulb because it has high melting point and it does not oxidize. Thus tungsten filament does not melt even when a large amount of heat is produced due to passage of current through the filament (via heating effect of current).
Q. Which gas is used in electric bulbs?
General service incandescent light bulbs over about 25 watts in rating are now filled with a mixture of mostly argon and some nitrogen, or sometimes krypton. While inert gas reduces filament evaporation, it also conducts heat from the filament, thereby cooling the filament and reducing efficiency.
Q. What is the resistivity of tungsten?
5.6 x10-8
Q. What material has the highest electrical resistance?
The material that has the highest electrical resistance is silver. After silver copper and gold are the materials that have the highest electrical resistance.
Q. What has the highest resistivity?
The various high resistivity materials (including alloys) are described below:
- Tungsten: (i) Hard metal.
- Carbon: (i) ρ = 1000 to 7000 μ ohm cm, α = – 0.0002 to – 0.0008.
- Nichrome or Brightray B: Composition:
- Nichrome V or Brightray C:
- Manganin:
- Constantan or Eureka:
- German Silver or Nickel Silver or Electrum:
- Nirosta:
Q. What is the resistance of a 20.0 m long piece of 12 gauge?
R=104mΩ
Q. What material has lowest electrical resistance?
The Resistivity of Various Materials A material with low resistivity means it has low resistance and thus the electrons flow smoothly through the material. For example, Copper and Aluminium have low resistivity. Good conductors have less resistivity. Insulators have a high resistivity.
Q. What is the resistance of steel?
Table of Resistivity and Conductivity at 20°C
| Material | ρ (Ω•m) at 20 °C Resistivity | σ (S/m) at 20 °C Conductivity |
|---|---|---|
| Carbon steel | (10 10) | 1.43×10 −7 |
| Lead | 2.2×10 −7 | 4.55×10 6 |
| Titanium | 4.20×10 −7 | 2.38×10 6 |
| Grain oriented electrical steel | 4.60×10 −7 | 2.17×10 6 |