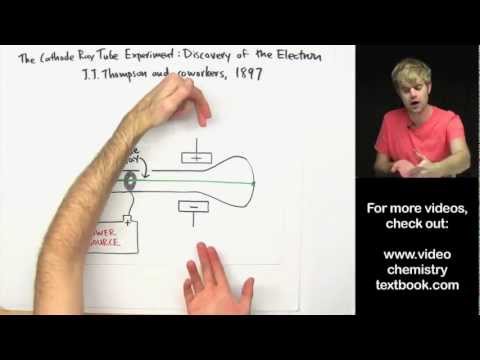
Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.”
Q. What was the conclusion of the cathode ray experiment?
Conclusion. After completing the experiment J.J. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. This theory further helped physicists in understanding the structure of an atom.
Table of Contents
- Q. What was the conclusion of the cathode ray experiment?
- Q. What did JJ Thomson conclude about the nature of the cathode ray particles based on this observation of the deflection of the cathode ray passing between the two plates?
- Q. What was JJ Thomson’s important discovery about cathode rays in 1897?
- Q. What was Ernest Rutherford’s experiment?
- Q. What did the gold foil experiment prove?
- Q. Why was the gold foil thin?
- Q. Why was Rutherford’s gold foil experiment important?
- Q. What is the smallest thing you can see with your eyes?
Q. What did JJ Thomson conclude about the nature of the cathode ray particles based on this observation of the deflection of the cathode ray passing between the two plates?
In order to determine if the cathode ray consisted of charged particles, Thomson used magnets and charged plates to deflect the cathode ray. He concluded that electrons were negatively charged subatomic particles present in atoms of all elements.
Q. What was JJ Thomson’s important discovery about cathode rays in 1897?
In 1897, J.J. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. He demonstrated that cathode rays were negatively charged. In addition, he also studied positively charged particles in neon gas.
Q. What was Ernest Rutherford’s experiment?
Ernest Rutherford’s most famous experiment is the gold foil experiment. A beam of alpha particles was aimed at a piece of gold foil. Most alpha particles passed through the foil, but a few were scattered backward. This showed that most of the atom is empty space surrounding a tiny nucleus.
Q. What did the gold foil experiment prove?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
Q. Why was the gold foil thin?
It was already known then that the range of the alpha particles that Rutherford worked with was not very large in gold. So if he wanted to see any effects on the alpha particles that passed through he had to produce a very thin gold foil.
Q. Why was Rutherford’s gold foil experiment important?
Rutherford’s experiment showed that atoms consisted of a dense mass which was surrounded by mostly empty space – the nucleus! The conclusion that could be formed from this result was that atoms had an inner core which contained most of the mass of an atom and was positively charged.
Q. What is the smallest thing you can see with your eyes?
Experts believe that the naked eye — a normal eye with regular vision and unaided by any other tools — can see objects as small as about 0.1 millimeters.