Q. Why do some atoms prefer to lose electrons?
The main force that keeps electrons in atoms is the electrical attraction between the electrons and the protons in the nucleus and so, if it is more energetically favourable to lose that electron in order to form a bond, then that is what will happen.
Q. Why is it easier for elements to lose electrons going down a group?
Tendency of an element to lose electrons increases when we go down the group. This is because atomic size increases when we go down the group. And force of attraction of nucleus for the valence electrons decreases down the group. This make easier for an element to lose electrons….
Table of Contents
- Q. Why do some atoms prefer to lose electrons?
- Q. Why is it easier for elements to lose electrons going down a group?
- Q. Why do atoms get smaller as you move down a group?
- Q. Why atoms tend to gain or lose electron?
- Q. Which of the following elements has maximum number of valence electrons?
- Q. Which one will have the maximum number of valence * CL Si Al P?
- Q. Which element in group 14 has the largest atomic radius?
- Q. Which has the largest radius K 1 or MG 2?
- Q. Is magnesium bigger than potassium?
Q. Why do atoms get smaller as you move down a group?
Moving from left to right across a period, atoms become smaller as the forces of attraction become stronger. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. This is caused by the decrease in atomic radius.
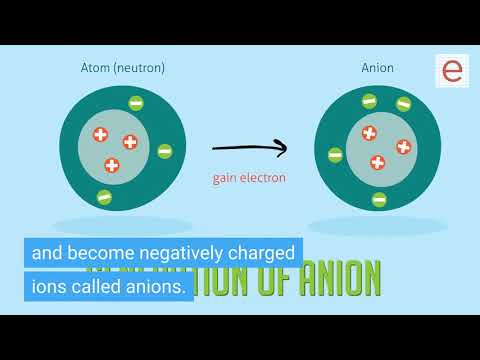
Q. Why atoms tend to gain or lose electron?
Atoms tend to gain or loss electrons in order to attain their nearest noble gas configuration and the completion of their octate helps them gain stability.
Q. Which of the following elements has maximum number of valence electrons?
Thus, phosphorus has the maximum number of valence electrons, i.e., 5.
Q. Which one will have the maximum number of valence * CL Si Al P?
Hence phosphorus has maximum number of valence electrons.
Q. Which element in group 14 has the largest atomic radius?
Carbon Family – Group 14 – Element Facts
| C | Sn | |
|---|---|---|
| atomic radius (pm) | 77 | 140 |
| ionic radius (pm) | 260 (C 4-) | 118 (Sn 2+) |
| usual oxidation number | +3, -4 | +2, +4 |
| hardness (Mohs) | 10 (diamond) | 1.5 |
Q. Which has the largest radius K 1 or MG 2?
K+ has the largest radius.
Q. Is magnesium bigger than potassium?
As we can see, only Potassium has four electron shells, therefore Potassium has the largest atomic size. Interestingly enough, Sodium has a larger atomic radius then magnesium, despite having less electrons and protons.