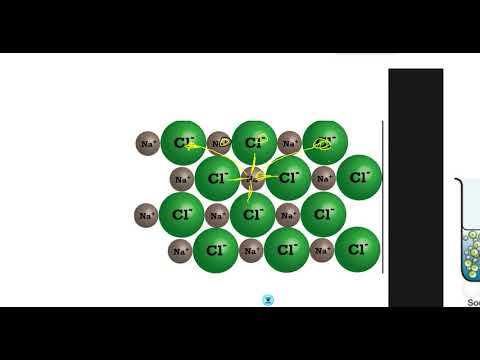
Q. Why do some substances conduct electricity when dissolved in water?
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the oppositely charged electrode.
Q. Why does a solution not conduct electricity when dissolved in water?
Urea solution does not conduct electricity because it is a covalent compound.
Table of Contents
- Q. Why do some substances conduct electricity when dissolved in water?
- Q. Why does a solution not conduct electricity when dissolved in water?
- Q. How do you know if a solution will conduct electricity?
- Q. Which one of the solution will not conduct electricity?
- Q. Does salt dissolve electricity in water?
- Q. What will happen if we add salt to distilled water?
- Q. Is rain water an insulator?
- Q. Is rain water good conductor of electricity?
- Q. Can electricity pass through pure water?
- Q. Is kerosene a conductor of electricity?
- Q. Is Sulphuric acid a good conductor of electricity?
- Q. Can pure H2SO4 conduct electricity?
- Q. Can H2SO4 conduct electricity in water?
- Q. Is copper sulphate is a bad conductor of electricity?
- Q. Is aluminum a good conductor of electricity?
- Q. Is plastic a good or bad conductor of electricity?
Q. How do you know if a solution will conduct electricity?
Metals conduct electricity, so if there is just the symbol of a metal, eg Mg(s), then it will conduct, even in the solid or liquid state. 2. Ionic compounds conduct when they are dissolved in water, or are melted. Ionic compounds always start with a metal.
Q. Which one of the solution will not conduct electricity?
Electrolytes are salts or molecules that ionize completely in solution. Nonelectrolytes do not dissociate into ions in solution; nonelectrolyte solutions do not, therefore, conduct electricity.
Q. Does salt dissolve electricity in water?
When an acid, a base, or a salt is dissolved in water, the molecules break into electrically charged particles called ions. Solutions with ions conduct electricity. Because pure water has few ions, it is a poor conductor. Uncharged molecules that dissolve in water, like sugar, do not conduct electricity.
Q. What will happen if we add salt to distilled water?
Distilled water is pure and free of salts; thus it is a very poor conductor of electricity. By adding ordinary table salt (NaCl) to distilled water, it becomes an electrolyte solution, able to conduct electricity.
Q. Is rain water an insulator?
Yes. While pure water is an electric insulator, rainwater is not pure water, even before it hits the ground.
Q. Is rain water good conductor of electricity?
The availability of ions is very important to conduct electricity. These gases dissolve in water to form some kind of acids like carbonic acids which dissociates to give ions. Thus rainwater is conducting electricity while distilled water is not conducting electricity.
Q. Can electricity pass through pure water?
Well actually, pure water is an excellent insulator and does not conduct electricity. The thing is, you won’t find any pure water in nature, so don’t mix electricity and water.
Q. Is kerosene a conductor of electricity?
Petrol and kerosene oil have very low electrical conductivity and hence they are poor electrical conductors (i.e. insulators).
Q. Is Sulphuric acid a good conductor of electricity?
Sulfuric acid is very popular for its high electrical conductivity. It conducts electricity very well because of the dissociation that happens through its protonation.
Q. Can pure H2SO4 conduct electricity?
Answer. Sulphuric Acid is a conductor of electricity because it furnish h+ ions in aqueous solution but h2so4 gas is not conductor of electricity because it doesn’t furnish h+ions.
Q. Can H2SO4 conduct electricity in water?
When H2SO4 desolve in water it will be ionized to form 2H+ and SO4 2- ions these ions free to move in solution due to this these free ions into the solution it will be conduct electricity .
Q. Is copper sulphate is a bad conductor of electricity?
Glass is usually an insulator and has electrons held tightly which means they aren’t shared between other atoms, so it is a poor conductor of electricity whereas copper is a metal and has free electrons in it and therefore it is a good conductor of electricity. Hence, the given statement is false.
Q. Is aluminum a good conductor of electricity?
Most metals are considered to be good conductors of electrical current. Copper is just one of the more popular materials that is used for conductors. Other materials that are sometimes used as conductors are silver, gold, and aluminum. Aluminum and most other metals do not conduct electricity quite as good as copper.
Q. Is plastic a good or bad conductor of electricity?
Plastics are usually a bad conductor of heat and electricity which means that it cannot conduct electricity or heat when passed through it. Complete answer: -Conductors are defined as those compounds which can conduct heat and electricity.