Q. Why do we use rectisol process?
Rectisol is used most often to treat synthesis gas (primarily hydrogen and carbon monoxide) produced by gasification of coal or heavy hydrocarbons, as the methanol solvent is well able to remove trace contaminants such as ammonia, mercury, and hydrogen cyanide usually found in these gases.
Q. What is Selexol used for?
An acid gas removal solvent often used for total acid gas removal. A formulated amine solvent used in gas treating applications to selectively remove H2S. A formulated amine solvent used to selectively remove H2S to very low levels in tail gas treating processes.
Table of Contents
- Q. Why do we use rectisol process?
- Q. What is Selexol used for?
- Q. What is Sulfinol?
- Q. What is a Rectisol process?
- Q. What is a Selexol unit?
- Q. What is mercaptan in natural gas?
- Q. How do I get rid of mercaptan?
- Q. Is Selexol flammable?
- Q. Why is mercaptan added to LPG?
- Q. What is mercaptan made from?
- Q. Is mercaptan flammable?
- Q. Which is the chemical solvent in the sulfinol process?
- Q. How is the sulfinol process different from the amine process?
- Q. When do you use the sulfinol D process?
- Q. Why is sulfinol used in gas treating plants?
Q. What is Sulfinol?
Sulfinol is an integrated, dual approach to acid gas removal and trace sulphur and mercaptans scrubbing. Sulfinol hybrid solvents enable simple all-in-one sour gas treatment, reducing equipment count and solvent rates significantly.
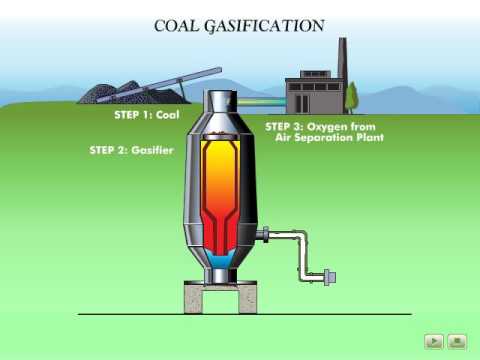
Q. What is a Rectisol process?
RECTISOL® is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures. RECTISOL® is therefore frequently used to purify shifted, partially shifted or unshifted gas downstream residue oil-, coal- or lignite gasification.
Q. What is a Selexol unit?
Selexol is the trade name for an acid gas removal solvent that can separate acid gases such as hydrogen sulfide and carbon dioxide from feed gas streams such as synthesis gas produced by gasification of coal, coke, or heavy hydrocarbon oils.
Q. What is mercaptan in natural gas?
Mercaptan is also known as methanethiol and is a harmless but pungent-smelling gas which has been described as having the stench of rotting cabbages or smelly socks. It is often added to natural gas, which is colourless and odourless, to make it easier to detect.
Q. How do I get rid of mercaptan?
Oxidation is the only method that completely eliminates mercaptan odors. Precipitation technology may adsorb some mercaptans, but may readily release the adsorbed molecules. Removal of sulfide by precipitation often allows the odor from mercaptans to be more apparent and equally offensive.
Q. Is Selexol flammable?
SelexolTM was discovered to be a low toxic and flammable solvent in contrast to Rectisol® which may result to death upon inhalation.
Q. Why is mercaptan added to LPG?
In the case of LPG to detect the leakage with the smell odorant is added. LPG is odorless due to this we need to add some substance which has its odor so that if gas leaks we can detect. Hence, to detect the leakage, ethyl mercaptan is added to LPG.
Q. What is mercaptan made from?
Versatility of Mercaptan Mercaptan is a non-toxic substance that is made of carbon, hydrogen, and sulfur. Because it’s regularly found in nature as a waste product for both animals and humans, mercaptans are also organic and extremely foul-smelling.
Q. Is mercaptan flammable?
* Breathing Ethyl Mercaptan can irritate the nose and throat. * Repeated or long term exposure to Ethyl Mercaptan may damage the red blood cells causing anemia. * Ethyl Mercaptan is a HIGHLY FLAMMABLE LIQUID or GAS and a DANGEROUS FIRE HAZARD.
Q. Which is the chemical solvent in the sulfinol process?
The sulfinol process uses a mixture of solvents allowing it to behave as both a chemical and physical solvent process. The solvent is composed of sulfolane, diisopropanolamine (DIPA), and water. The sulfolane acts as the physical solvent, while DIPA acts as the chemical solvent.
Q. How is the sulfinol process different from the amine process?
The main difference is that while the conventional amine process employs a fairly diluted concentration of amine in water, removing the acid gas by chemical reaction, the Sulfinol system uses a mixture of highly concentrated amine and a physical solvent removing the acid gases by physical and chemical reactions.
Q. When do you use the sulfinol D process?
The Sulfinol-D process is primarily used in cases where selective removal of hydrogen sulfide is not of primary concern, but where partial removal of organic sulfur compounds (mercaptans, RSH, and carbon disulfide) is desired, typically in natural gas and refinery applications.
Q. Why is sulfinol used in gas treating plants?
Sulfinol processes are streamlined, all-in-one solutions to maximise results. Enhanced solubility provides quicker mercaptan removal and lower hydrocarbon co-absorption. Shell Catalysts & Technologies has seen firsthand the effects of gas-treating plants switching to the Sulfinol process.