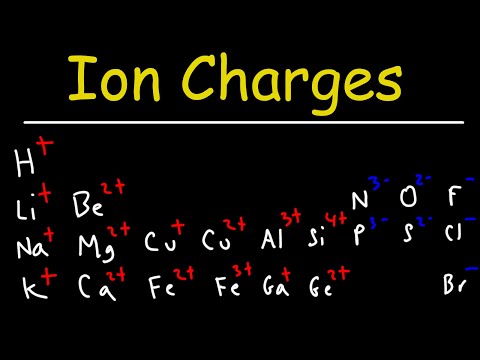Q. Why does Al have a +3 charge?
The charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons. The valence shell of aluminum has three electrons, and per the octet rule, these three electrons are lost resulting in just 10 electrons and 13 protons.
Q. What is the name of Al 3?
aluminum cation
Table of Contents
- Q. Why does Al have a +3 charge?
- Q. What is the name of Al 3?
- Q. What is the oxidation of Hg?
- Q. What is the oxidation number of Hg in Hg2 2+?
- Q. What type of bond is HG?
- Q. What does Hg bond mean?
- Q. Is Hg a metallic bond?
- Q. Is Hg a covalent?
- Q. Is Br2 a covalent network solid?
- Q. What is the shape of Br2?
- Q. What kind of covalent bond is Br2?
- Q. What type of solid is Br2?
- Q. What type of solid is KBR?
- Q. Which type of solid is P4?
- Q. Which type of solid is silver?
- Q. What is the name for P4?
Q. What is the oxidation of Hg?
The Hg oxidation level was 91.1 ± 2.2% at 37.8 °C.
Q. What is the oxidation number of Hg in Hg2 2+?
+2
Q. What type of bond is HG?
covalent bond
Q. What does Hg bond mean?
High-yield bonds (also called junk bonds) are bonds that pay higher interest rates because they have lower credit ratings than investment-grade bonds. High-yield bonds are more likely to default, so they must pay a higher yield than investment-grade bonds to compensate investors.
Q. Is Hg a metallic bond?
Metallic bonding is the main type of chemical bond that forms between metal atoms. Metallic bonds are seen in pure metals and alloys and some metalloids. For example, the mercurous ion (Hg22+) can form metal-metal covalent bonds.
Q. Is Hg a covalent?
Mercury undergoes weak covalent bonding with its partner X in most cases (exceptions: X=alkali atoms, which lead to van der Waals bonding) although the BDEs are mostly smaller than 12 kcal molА1. Bonding is weakened by 1) a singly occupied desta- bilized s*-HOMO and 2) lone pair repulsion.
Q. Is Br2 a covalent network solid?
If that’s the case, why does Br2 (2x Br) form a molecular solid when the two are bonded by covalent bonds? 2 carbon atoms are covalently bonded yet they make a covalent network solid.
Q. What is the shape of Br2?
Regarding the polarity of Br2, you must read out an article on the polarity of Br2. Due to the presence of atoms of the same element, the molecular geometry of Dibromine is Linear and the compound has a symmetrical shape.
Q. What kind of covalent bond is Br2?
Having the same electronegativity of both atoms, both share an equal proportion of charge. The atoms forming a covalent bond having equal electronegativity are nonpolar in nature. Therefore, the Br2 molecule is a nonpolar molecule.
Q. What type of solid is Br2?
molecular solids
Q. What type of solid is KBR?
Potassium bromide is a metal bromide salt with a K(+) counterion.
Q. Which type of solid is P4?
molecular solid
Q. Which type of solid is silver?
metallic solid
Q. What is the name for P4?
tetraphosphorus Phosphorus