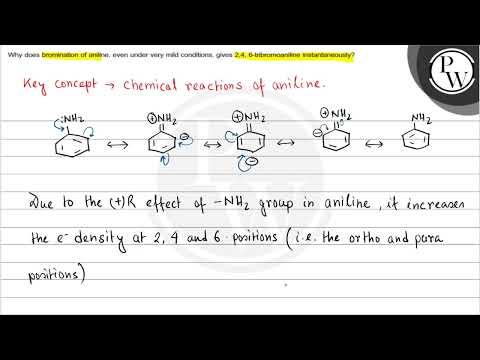
Q. Why does bromination of aniline even under very mild conditions gives 2 4 6 Tribromoaniline instantaneously?
Why does bromination of aniline, even under very mild conditions, gives 2,4,6-tribromoaniline instantanesouly? due to strong electron-donating effect of the -NH2 group, the electron density increases at the o-,p-positions.
Q. When aniline forms 2 4 6 Tribromo aniline on reaction with bromine water it undergoes?
Principle: Aniline undergoes nucleophilic substitution with bromine, even in cold. The bromine atoms enter at the two ortho positions and the para position with the formation of 2,4,6-tribromoaniline.
Table of Contents
- Q. Why does bromination of aniline even under very mild conditions gives 2 4 6 Tribromoaniline instantaneously?
- Q. When aniline forms 2 4 6 Tribromo aniline on reaction with bromine water it undergoes?
- Q. What is the Colour of Tribromoaniline?
- Q. Which apparatus are used in the preparation of 2 4 6 Tribromoaniline?
- Q. How will you convert aniline into phenol?
- Q. How is aniline converted to benzoic acid?
- Q. How do you convert chlorobenzene to aniline?
- Q. Can be converted into aniline in presence of?
- Q. Which reagent must be added in order to get aniline from chlorobenzene in one step?
- Q. How will you convert nitrobenzene to aniline?
- Q. How does aniline reacts with cold nitrous acid?
- Q. How do you convert aniline to phenyl Isocyanide?
- Q. How would you convert aniline to 4 aniline?
- Q. How is ethane converted to Bromoethene?
- Q. How is toluene converted to benzyl alcohol?
- Q. Is ethane a carcinogen?
- Q. What are the uses of ethane ethylene in our daily lives?
Q. What is the Colour of Tribromoaniline?
By reacting bromine with aniline in water, a white precipitate immediately forms and that is 2,4,6-tribromoaniline.
Q. Which apparatus are used in the preparation of 2 4 6 Tribromoaniline?
1.9. The substitutions take place in ortho and one para position to gives 2’4’6 Tribromoaniline. Apparatus required: Conical flask, beaker and glass rod. Procedure: Take 5 ml of aniline and 19 ml of glacial acetic acid in a flask.
Q. How will you convert aniline into phenol?
Thus, we can convert aniline to phenol by first treating aniline with sodium nitride and hydrochloric acid which gives benzene diazonium salt which in reaction with water gives phenol. Note: In the reaction, phenol is prepared. Thus, it is a preparation reaction of phenol.
Q. How is aniline converted to benzoic acid?
This reaction involves three steps :
- Step 1 : Conversion of aniline to benzene diazonium salt. When aniline react with in presence of hydrochloric acid to give benzene diazonium salt as a product.
- Step 2 : Conversion of benzene diazonium salt to cyanobenzene.
- Step 3 : Conversion of cyanobenzene to benzoic acid.
Q. How do you convert chlorobenzene to aniline?
> A mixture of chlorobenzene and ammonia is passed through a heated tube and then the tube is cooled and washed with water to remove the byproduct ammonium chloride and by fractional distillation the organic compound i.e. aniline is recovered.
Q. Can be converted into aniline in presence of?
Nitrobenzene is an aromatic nitro compound and aniline is its corresponding aromatic primary amine. So, nitrobenzene can be converted into aniline by reduction with an active metal like iron, tin, zinc etc. and concentrated hydrochloric acid.
Q. Which reagent must be added in order to get aniline from chlorobenzene in one step?
Chlorobenzene is soluble in water and it is volatile in nature. The conversion of aniline to chlorobenzene takes place as follows: a) Aniline is initially reacted with sodium nitrite and hydrochloric acid at low temperature i.e 0−4∘C. This gives diazonium ion or a diazonium salt.
Q. How will you convert nitrobenzene to aniline?
Nitrobenzene is reduced to aniline by Sn and concentrated HCl. Instead of Sn, Zn or Fe also can be used. Aniline salt is given from this reaction. Then aqueous NaOH is added to the aniline salt to get released aniline.
Q. How does aniline reacts with cold nitrous acid?
(i) Primary aromatic amines such as aniline react with nitrous acid under ice cold conditions (273-278 K) to form benzene diazonium salt. The reaction is known as diazotization reaction. (i) If a clear solution is obtained , then it is a primary amine.
Q. How do you convert aniline to phenyl Isocyanide?
1 Answer. Aniline on heating with Chloroform in the presence of KOH gives phenyl isocyanide. This reaction is known as Carbylamine Reaction. It’s also used as a test for primary amines.
Q. How would you convert aniline to 4 aniline?
In the presence of pyridine, first treat aniline with (CH3CO2)2O. The product will be replaced by COCH3 with one H of -NH2. Use the Nitrating mix to treat this.
Q. How is ethane converted to Bromoethene?
Ethane is converted to bromoethene by first reacting ethane with bromine in presence of U.V light to form bromoethane. Then bromoethane is reacted with alcoholic potassium hydroxide to form ethene.
Q. How is toluene converted to benzyl alcohol?
The conversion of toluene into benzyl alcohol can be achieved in two steps. Free radical chlorination of toluene with chlorine in presence of ultraviolet light or heat give benzyl chloride. One hydrogen atom of methyl group of toluene is replaced with chlorine atom. A molecule of hydrogen chloride is eliminated.
Q. Is ethane a carcinogen?
It has been postulated that ethene exposures, especially in urban areas, may be expected to lead to a lifetime cancer risk in humans.
Q. What are the uses of ethane ethylene in our daily lives?
Hydrocarbon gas liquids have many uses
| HGL | Uses | End-use products |
|---|---|---|
| Ethane | Petrochemical feedstock for ethylene production; power generation | Plastics; anti-freeze; detergents |
| Propane | Fuel for space heating, water heating, cooking, drying, and transportation; petrochemical feedstock | Fuel for heating, cooking, and drying; plastics |