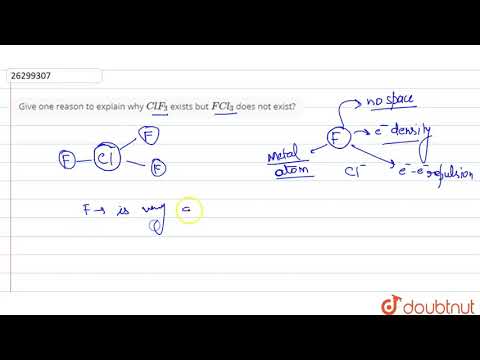
Answer. Flourine doesnot possess d orbitals, so it cannot expand its octet to bond with three chlorine atoms and form FCl3. Chlorine on the other hand has empty d orbitals and thus it can expand its octet to bond with three flourine atoms and form ClF3.
Q. What existed means?
1. to have actual being; be. 2. to have life or animation; live. 3. to continue to be or live: Belief in magic still exists. 4. to have being in a specified place or under certain conditions; be found; occur. 5. to achieve the basic needs of existence, as food and shelter.
Table of Contents
Q. Does clbr7 exist?
Answer. Answer: Explanation: ClF7 doesn’t exist because chlorine is a smaller atom as compared to iodine, so it cannot “pack” the seven fluorine atoms around itself due to expulsion between the electron clouds of F atoms.
Q. Why SH6 do not exist?
This molecule is octahedral in shape. Hydrogen is a very weak oxidizing agent as compared to fluorine this means that hydrogen is not able to oxidise sulphur to its maximum oxidation state. Hence, SH6 does not exist.
Q. Does IBr7 exist?
Answer: The existence of IF7 and non-existence of IBr7 can be explained on the basis of electronegativity and the size of atoms. – Due to this, it can form 7 bonds with iodine very easily and form IF7.
Q. Why NCl5 is not formed?
PCl5 forms five bonds by using the d-orbitals to “expand the octet” and have more places to put bonding pairs of electrons. NCl5 does not exist because there are no d-orbitals in the second energy level. Therefore there is no way to arrange five pairs of bonding electrons around a nitrogen atom.
Q. Which species does not exist?
(CCl6)2− species does not exist. Carbon cannot accept 6Cl−, since it has no vacant d-orbitals.
Q. How is PCl5 formed?
Phosphorus penta chloride is one of the important compounds of phosphorus and chlorine. Preparation: Phosphorus Penta chloride is prepared by passing an excess of dry chlorine into liquid trichloride. Chlorine reacts with phosphorus trichloride and solid phosphorus penta chloride is formed.
Q. What is the Lewis dot structure of PCl5?
Drawing the Lewis Structure for PCl There are a total of 40 valence electrons in the PCl5 Lewis structure. Remember when you draw the Lewis structure for PCl5 that Phosphorous (P) is in Period 3 on the Periodic table. This means that it can hold more than 8 valence electrons.
Q. Is PCl5 solid or liquid?
Phosphorus pentachloride is a greenish-yellow crystalline solid with an irritating odor. It is decomposed by water to form hydrochloric and phosphoric acid and heat.
Q. Why PCl3 is not a Lewis acid?
P(CH3)3 has an electron-rich phosphorus atom with a lone pair to share with a Lewis acid. The electronegative chlorine atoms pull electron density away from the phosphorus atom in PCl3. As such, the phosphorus atom cannot readily share its lone pair, and it is not a good Lewis base.