Hemoglobin with bound carbon dioxide and hydrogen ions is carried in the blood back to the lungs, where it releases the hydrogen ions and carbon dioxide and rebinds oxygen. Thus, hemoglobin helps to transport hydrogen ions and carbon dioxide in addition to transporting oxygen.
Q. What type of blood cell is responsible for blood clots?
The main job of platelets, or thrombocytes, is blood clotting. Platelets are much smaller in size than the other blood cells. They group together to form clumps, or a plug, in the hole of a vessel to stop bleeding.
Table of Contents
- Q. What type of blood cell is responsible for blood clots?
- Q. Which has the greatest effect on the ability of blood to transport oxygen?
- Q. What increases the affinity of oxygen to hemoglobin?
- Q. How does Haemoglobin load and unload oxygen in the body?
- Q. What causes left shift in oxyhemoglobin curve?
- Q. Which would make the oxygen hemoglobin curve shift right?
- Q. How does pH affect oxygen dissociation curve?
- Q. What affects the oxygen dissociation curve?
- Q. Does pH affect oxygen levels?
- Q. Does higher pH mean more oxygen?
- Q. How does a low pH affect Haemoglobin?
- Q. Why does pH increase when co2 decreases?
- Q. How does hemoglobin help maintain blood pH?
Q. Which has the greatest effect on the ability of blood to transport oxygen?
Carbon dioxide levels, blood pH, and body temperature affect oxygen-carrying capacity (Figure 2). When carbon dioxide is in the blood, it reacts with water to form bicarbonate (HCO−3) and hydrogen ions (H+). As the level of carbon dioxide in the blood increases, more H+ is produced and the pH decreases.
Q. What increases the affinity of oxygen to hemoglobin?
Carbon Monoxide: Binding of one CO molecule to hemoglobin increases the affinity of the other binding spots for oxygen, leading to a left shift in the dissociation curve.
Q. How does Haemoglobin load and unload oxygen in the body?
The process by which hemoglobin binds oxygen to form oxyhemoglobin is called loading. That’s what happens in the lungs. Once in the metabolizing tissues, oxyhemoglobin is unloaded as oxygen is released and diffuses into the plasma and ultimately our cells.
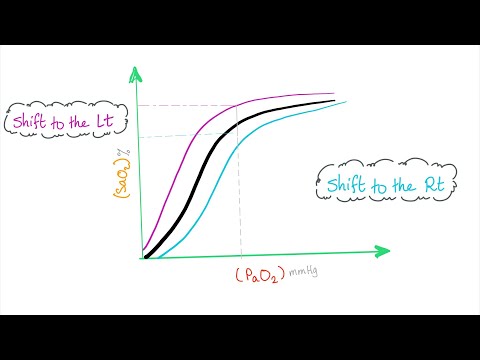
Q. What causes left shift in oxyhemoglobin curve?
A rightward shift of the curve indicates that hemoglobin has a decreased affinity for oxygen, thus, oxygen actively unloads. A shift to the left indicates increased hemoglobin affinity for oxygen and an increased reluctance to release oxygen.
Q. Which would make the oxygen hemoglobin curve shift right?
An increased concentration of BPG in red blood cells favours formation of the T (taut or tense), low-affinity state of hemoglobin and so the oxygen-binding curve will shift to the right.
Q. How does pH affect oxygen dissociation curve?
In contrast, an elevated (= alkaline or basic) blood plasma pH of 7.6 causes the O2-Hb saturation curve to shift about 15% to the left of normal. As blood plasma pH decreases (= becomes more acidic), H+ ions increasingly bind to hemoglobin amino acids, which lessens hemoglobin’s affinity for O2.
Q. What affects the oxygen dissociation curve?
The oxygen–hemoglobin dissociation curve can be displaced such that the affinity for oxygen is altered. Factors that shift the curve include changes in carbon dioxide concentration, blood temperature, blood pH, and the concentration of 2,3-diphosphoglycerate (2,3-DPG).
Q. Does pH affect oxygen levels?
A minor increase in pH levels can cause a oligotrophic (rich in dissolved oxygen) lake to become eutrophic (lacking dissolved oxygen). Even minor pH changes can have long-term effects.
Q. Does higher pH mean more oxygen?
We hypothesize that the dissolved oxygen levels decrease due to increasing levels of pH, thus inhibiting aquatic life that keeps dissolved oxygen levels high.
Q. How does a low pH affect Haemoglobin?
A lower pH in the blood is suggestive of an increased carbon dioxide concentration which in turn, is suggestive of a more active tissue that requires more oxygen. According to Bohr, the lower pH will cause hemoglobin to deliver more oxygen.
Q. Why does pH increase when co2 decreases?
Since carbon dioxide reacts with water to form carbonic acid, an increase in CO2 results in a decrease in blood pH, resulting in hemoglobin proteins releasing their load of oxygen. Conversely, a decrease in carbon dioxide provokes an increase in pH, which results in hemoglobin picking up more oxygen.
Q. How does hemoglobin help maintain blood pH?
Hemoglobin is the principal protein inside of red blood cells and accounts for one-third of the mass of the cell. During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. This buffering helps maintain normal pH.