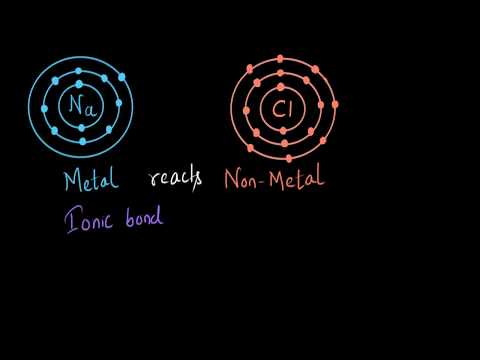
Q. Why ionic compounds form from metals and nonmetals?
Ionic bonds form only between metals and nonmetals. That’s because metals “want” to give up electrons, and nonmetals “want” to gain electrons. It takes energy to remove valence electrons from an atom and form a positive ion. Energy is released when an atom gains valence electrons and forms a negative ion.
Q. Are ionic compounds made of metals and nonmetals?
Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds.
Table of Contents
- Q. Why ionic compounds form from metals and nonmetals?
- Q. Are ionic compounds made of metals and nonmetals?
- Q. Why can different numbers of metal and nonmetal atoms create ionic bonds together?
- Q. Why do metals form positive ions?
- Q. What two atoms form ionic bonds?
- Q. What produces an ionic bond?
- Q. What are the examples of Electrovalent bond?
- Q. What are called Electrovalent compounds?
- Q. What is Electrovalent and covalent bond?
- Q. Which compound is Electrovalent?
- Q. Which is strongest bond in chemistry?
- Q. What are the properties of Electrovalent compounds?
- Q. Why ionic reactions are fast?
- Q. What is an Electrovalent salt?
- Q. Why are Electrovalent compounds soluble in water?
- Q. Why is Electrovalent?
- Q. Are all Electrovalent compounds soluble in water?
- Q. Is water is an Electrovalent compound?
- Q. Is NaCl an Electrovalent compound?
- Q. Why are ionic compounds insoluble in kerosene?
Q. Why can different numbers of metal and nonmetal atoms create ionic bonds together?
The positively charged ions and the negatively charged ions create an electrostatic attraction which results to the formation an ionic bond. Therefore, different number of metal and non-metal atoms can create ionic bonds together.
Q. Why do metals form positive ions?
Metal atoms lose electrons from their outer shell when they form ions: the ions are positive, because they have more protons than electrons. the ions formed have full outer shells.
Q. What two atoms form ionic bonds?
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal.
Q. What produces an ionic bond?
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
Q. What are the examples of Electrovalent bond?
An electrovalent bond is formed when a metal atom transfers one or more electrons to a non-metal atom. Some other examples are: MgCl2, CaCl2, MgO, Na2S, CaH2, AlF3, NaH, KH, K2O, KI, RbCl, NaBr, CaH2 etc.
Q. What are called Electrovalent compounds?
Ionic compounds are formed by the transfer of electron. Due to this a cation and anion exist in ionic compounds and there is a force of attraction exist between these positive and negatively charged ions and these forces are electrovalent in nature. So the ionic compounds are called electrovalent compounds.
Q. What is Electrovalent and covalent bond?
1 ) Electrovalent compounds are formed by complete rtransfer of electrons while covalent compounds are formed by sharing of electrons between 2 atoms.
Q. Which compound is Electrovalent?
The compounds which contain ionic or electrovalent bonds are Electrovalent or Ionic Compounds. Mainly electrovalent compounds are formed due to the reaction between highly electropositive and highly electronegative atoms.
Q. Which is strongest bond in chemistry?
covalent bond
Q. What are the properties of Electrovalent compounds?
The general characteristics of electrovalent compounds are:
- (i) Electrovalent compounds are mostly crystalline in nature.
- (ii) Electrovalent compounds form hard crystals.
- (iii) Electrovalent compounds have high density with high melting and boiling points.
- (iv) Electrovalent compounds are soluble in polar solvents.
Q. Why ionic reactions are fast?
Ionic compounds in solution react faster than molecular compounds. This is because Ionic compounds break apart to form free ions. Therefore, there are no bonds to break so the reaction is fast. …
Q. What is an Electrovalent salt?
Electrovalent or ionic compounds are characterized by having ionic bonds between oppositely charged ions. This ionic bond consists of the electrostatic attraction between the two opposite charges.
Q. Why are Electrovalent compounds soluble in water?
Electrovalent compounds dissolve in polar solvent like water because the forces of attraction between positive and negative charges become weak in water. But since covalent compound are made up of molecules, they do not ionize in water and hence do not dissolve in water.
Q. Why is Electrovalent?
The crystals of electrovalent compounds are made up of crystal lattice containing oppositely charged ions. Each cation is surrounded by a definite number of anions and vice-versa. Their is a great electrostatic force of attraction among these oppositely charged ions and as a result, ionic compounds form hard crystals.
Q. Are all Electrovalent compounds soluble in water?
Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc. They have high melting and boiling points. This is because a considerable amount of energy is required to break the strong inter-ionic attraction between the molecules.
Q. Is water is an Electrovalent compound?
Solution. As water is a polar compound, it decreases the electrostatic forces of attraction, resulting in free ions in the aqueous solution. Hence, electrovalent compounds dissolve. Organic solvents are non-polar; hence, these dissolve in non-polar covalent compounds.
Q. Is NaCl an Electrovalent compound?
Since NaCl compounds are also formed by the transfer of one electron thus, NaCl is an electrovalent compound. Hence, NaCl is an electrovalent compound.
Q. Why are ionic compounds insoluble in kerosene?
Soluble in water, Insoluble in kerosene Water breaks the ionic bond by hydrogen bonding, as, water itself has a more ionic bond and polar in nature. Hence, can not dissolve them, and they all have covalent bonds and which are non-polar in nature.