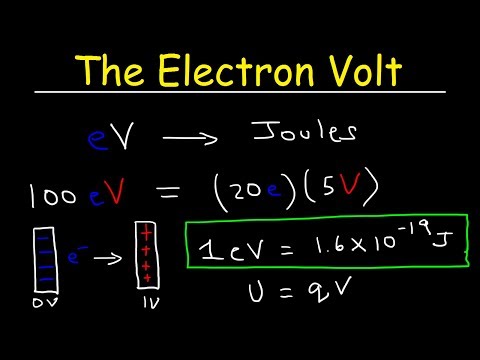
Q. Why is an electron volt A unit of energy?
Electron volt, unit of energy commonly used in atomic and nuclear physics, equal to the energy gained by an electron (a charged particle carrying unit electronic charge) when the electrical potential at the electron increases by one volt. The electron volt equals 1.602 × 10−12 erg, or 1.602 × 10−19 joule.
Q. Is electron volt bigger than Joule?
Electron volt in short Ev is the unit of energy. Joule is a derived unit of energy in the International System of units or SI units. Relation Between Ev And Joule is proportionate….Joule to eV.
Table of Contents
- Q. Why is an electron volt A unit of energy?
- Q. Is electron volt bigger than Joule?
- Q. What is the difference between Volt and electron volt?
- Q. What is the significance of electron volts?
- Q. What is electron volt simple definition?
- Q. What was the electron first called?
- Q. Who is father of Proton?
- Q. Who named Neutron?
- Q. What is the proton symbol?
- Q. Who has invented Proton?
- Q. What is discovered by Goldstein?
- Q. Can you see a proton?
- Q. Who invented nucleus?
- Q. What is the function of nucleus?
- Q. How was Nucleus Discovered?
- Q. Why is the nucleus positive in charge?
- Q. Which subatomic has least mass?
- Q. Which particle has lowest mass?
- Q. Which particle has least mass?
- Q. Which particle has the least mass * 1 point?
- Q. Why do electrons have the least mass?
| Energy in Joules | Energy in eV |
|---|---|
| 8 J | 4.993×1019 eV |
| 9 J | 5.617×1019 eV |
| 10 J | 6.242×1019 eV |
| 50 J | 3.121×1020 eV |
Q. What is the difference between Volt and electron volt?
Volt is a unit of electric potential, or electric potential difference, while electron volt is a unit of energy. Volt is a unit of electric potential, or electric potential difference, while electron volt is a unit of energy. Volt is not a measure of the amount of electrons.
Q. What is the significance of electron volts?
A unit of energy in nuclear physics is the electron volt, which is defined as the energy gained by an electron in rising through a potential difference of one volt. Because electron volts precisely measure such small quantities of energy, they rank as the unit of choice for nuclear and atomic physics.
Q. What is electron volt simple definition?
George Lebo, University of Florida: “An electron volt (eV) is the energy that an electron gains when it travels through a potential of one volt. You can imagine that the electron starts at the negative plate of a parallel plate capacitor and accelerates to the positive plate, which is at one volt higher potential.
Q. What was the electron first called?
corpuscles
Q. Who is father of Proton?
Ernest Rutherford
Q. Who named Neutron?
In May 1932 James Chadwick announced that the core also contained a new uncharged particle, which he called the neutron.
Q. What is the proton symbol?
p
Q. Who has invented Proton?
Proton
| The quark content of a proton. The color assignment of individual quarks is arbitrary, but all three colors must be present. Forces between quarks are mediated by gluons. | |
|---|---|
| Classification | Baryon |
| Discovered | Observed as H+ by Eugen Goldstein (1886). Identified in other nuclei (and named) by Ernest Rutherford (1917–1920). |
Q. What is discovered by Goldstein?
Anode ray
Q. Can you see a proton?
(PhysOrg.com) — What does a proton look like? The common answer to this question is that protons are much too small to scatter light, and since light is necessary for us to see things, protons do not “look” like anything.
Q. Who invented nucleus?
Ernest Rutherford’s
Q. What is the function of nucleus?
The nucleus controls and regulates the activities of the cell (e.g., growth and metabolism) and carries the genes, structures that contain the hereditary information.
Q. How was Nucleus Discovered?
In 1911, Rutherford, Marsden and Geiger discovered the dense atomic nucleus by bombarding a thin gold sheet with the alpha particles emitted by radium. From this observation, they concluded that almost all the atomic matter was concentrated in a tiny volume situated at the atome center, the atomic nucleus.
Q. Why is the nucleus positive in charge?
The nucleus has an overall positive charge as it contains the protons. Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral.
Q. Which subatomic has least mass?
electron
Q. Which particle has lowest mass?
Q. Which particle has least mass?
Q. Which particle has the least mass * 1 point?
Neutrinos
Q. Why do electrons have the least mass?
Electrons are usually depicted in drawings as much smaller than protons or neutrons because their mass is so much smaller. In fact, electron mass is so small that it is not counted in an atom’s mass.