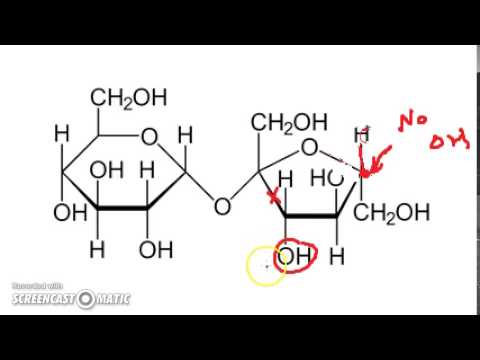
Q. Why is lactose a reducing sugar but sucrose is not?
Because the aglycone is a hemiacetal, lactose undergoes mutarotation. For the same reason lactose is a reducing sugar. The free aldehyde formed by ring opening can react with Benedict’s solution. Thus, a solution of lactose contains both the α and β anomer at the “reducing end” of the disaccharide.
Q. Is sucrose or lactose a reducing sugar?
It is a reducing sugar that is found in milk. Sucrose is composed of a molecule of glucose joined to a molecule of fructose by an α-1,β-2-glycosidic linkage.
Table of Contents
- Q. Why is lactose a reducing sugar but sucrose is not?
- Q. Is sucrose or lactose a reducing sugar?
- Q. Is xylose soluble in water?
- Q. Is xylose a hexose?
- Q. What is the formula of xylose?
- Q. Is c5h10o5 soluble in water?
- Q. How many carbons are in xylose?
- Q. Is C5H10O5 a sugar?
- Q. What is the chemical name of C6H12O6?
- Q. What is full form C6H12O6?
- Q. What is the chemical name for dextrose?
- Q. What is C2H5OH chemical name?
- Q. What is 95 ethanol called?
- Q. What is the chemical name of ethanol?
- Q. Which is better ethyl alcohol or isopropyl alcohol?
- Q. What is the best alcohol for disinfecting?
- Q. Is ethyl alcohol safe on skin?
Q. Is xylose soluble in water?
properties of xylose. Its molecular weight is 150.130 g/mole and its Melting point is153 °C. Its specific gravity is d204 is 1.525 and its refractive index nD20 is 1.517. Its solubility in water at 20 °C is 117 g per 100 ml of water and its crystals are white.
Q. Is xylose a hexose?
Greek: ξύλον, xylon, “wood”) is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group….Xylose.
| Names | |
|---|---|
| Related aldopentoses | Arabinose Ribose Lyxose |
| Related compounds | Xylulose |
Q. What is the formula of xylose?
C₆H₁₂O₆
Q. Is c5h10o5 soluble in water?
Solubility Soluble in water . Melting Point 160°C.
Q. How many carbons are in xylose?
five carbon atoms
Q. Is C5H10O5 a sugar?
Ribose is an organic compound with the formula C5H10O5; specifically, a pentose monosaccharide (simple sugar) with linear form H−(C=O)−(CHOH)4−H, which has all the hydroxyl groups on the same side in the Fischer projection. The term may refer to either of two enantiomers.
Q. What is the chemical name of C6H12O6?
D-glucose
Q. What is full form C6H12O6?
This monosaccharide has a chemical formula C6H12O6. It is also known as dextrose. D- glucose is the naturally occurring form of glucose.
Q. What is the chemical name for dextrose?
Q. What is C2H5OH chemical name?
ethanol
Q. What is 95 ethanol called?
The purity of rectified spirit has a practical limit of 95% ABV (95.6% by mass) when produced using conventional distillation processes, as a mixture of ethanol and water becomes a minimum-boiling azeotrope at this concentration.
Q. What is the chemical name of ethanol?
Q. Which is better ethyl alcohol or isopropyl alcohol?
Sanitizer Alcohol Percentage The World Health Organization suggests that 70% ethyl alcohol is superior to isopropyl alcohol against the influenza virus, however, both provide adequate germicidal properties. Ethanol is recommended at higher % concentration, usually 80%.
Q. What is the best alcohol for disinfecting?
Isopropyl alcohol, particularly in solutions between 60% and 90% alcohol with 10 – 40% purified water, is rapidly antimicrobial against bacteria, fungi, and viruses. Once alcohol concentrations drop below 50%, usefulness for disinfection drops sharply.
Q. Is ethyl alcohol safe on skin?
1. Topically applied ethanol (e.g. in the form of cosmetics or hand disinfectants) on un-lacerated human skin will not cause acute or systemic toxic effects, which can only occur if applied on damaged skin especially in children.