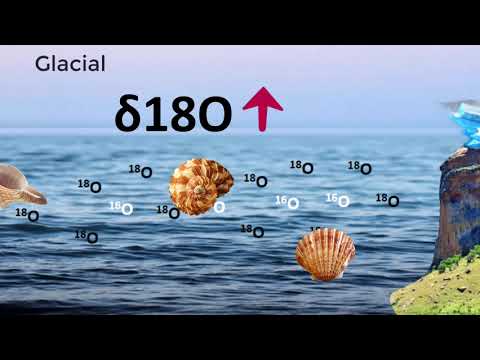
Since it is lighter than 18O, 16O evaporates first, so in warm, tropical areas, the ocean is high in 18O. Additionally, as water vapor condenses to form rain, water droplets rich in 18O precipitate first because it is heavier than 16O.
Q. Can you breathe oxygen 17?
Oxygen 17 is an isotope of oxygen. Oxygen-17 exists in nature as well and its possible you are currently breathing it and you do drink it ! Oxygen 17 is rare stable isotope of oxygen. It is having eight protons and nine neutrons; it amounts to 0.037% of the element in nature.
Table of Contents
- Q. Can you breathe oxygen 17?
- Q. How high can humans breathe?
- Q. Is oxygen-18 a stable isotope?
- Q. Is oxygen-18 stable or unstable?
- Q. What is the percentage of Br?
- Q. Is bromine stable or unstable?
- Q. Is bromination or chlorination faster?
- Q. Where is bromine found on Earth?
- Q. Why is bromine so selective?
- Q. Is iodine or bromine more reactive?
- Q. Which is more stable Cl2 or Br2?
Q. How high can humans breathe?
It is the lack of oxygen rather than the reduced air pressure that actually limits the height at which we can breathe. An elevation of about 20,000 feet above sea level is the maximum height at which sufficient oxygen exists in the air to sustain us.
Q. Is oxygen-18 a stable isotope?
Oxygen is a mixture of three stable isotopes: Oxygen-16 (99.76% at.), Oxygen-17 (0.04% at.) and Oxygen-18 (0.20% at.). The difference of mass numbers of isotopes affects their basic properties to a small extent, and therefore oxygen isotope composition of various natural compounds is approximately equal.
Q. Is oxygen-18 stable or unstable?
Isotopes of oxygen
| Isotope | Decay | |
|---|---|---|
| abundance | mode | |
| 16O | 99.76% | stable |
| 17O | 0.04% | stable |
| 18O | 0.20% | stable |
Q. What is the percentage of Br?
100.000%
Q. Is bromine stable or unstable?
Natural bromine is a mixture of two stable isotopes: bromine-79 (50.54 percent) and bromine-81 (49.46 percent). Of the 17 known radioactive isotopes of the element, bromine-77 has the longest half-life (57 hours).
Q. Is bromination or chlorination faster?
Since bromine has a lower reactivity, bromination requires a higher reaction temperature in order to run as fast as chlorination. In bromination at 98 °C, secondary C-H bonds react 250 times faster, while tertiary C-H bonds are attacked even 6300 times faster than primary C-H bonds.
Q. Where is bromine found on Earth?
Bromine is found naturally in the earth’s crust and in seawater in various chemical forms. Bromine can also be found as an alternative to chlorine in swimming pools. Products containing bromine are used in agriculture and sanitation and as fire retardants (chemicals that help prevent things from catching fire).
Q. Why is bromine so selective?
Bromination of alkanes occurs by a similar mechanism, but is slower and more selective because a bromine atom is a less reactive hydrogen abstraction agent than a chlorine atom, as reflected by the higher bond energy of H-Cl than H-Br.
Q. Is iodine or bromine more reactive?
Bromine is more reactive than iodine, but not as reactive as chlorine. Also, bromine has two isotopes: 79Br and 81Br. Bromine consists of bromide salts, which have been found in the sea.
Q. Which is more stable Cl2 or Br2?
Cl2 is more reactive because Cl- is more stable than Br-. In the case of Cl2 and Br2, Cl2 is more reactive because Cl- is more stable and there’s not as much pressure (where the equilibrium concept comes into play) to remain as Cl2.