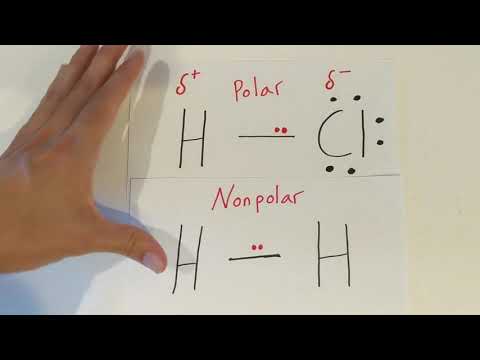
Q. Why is oxygen partially negative and hydrogen positive?
Because oxygen is more electronegative—electron-greedy—than hydrogen, the Ostart text, O, end text atom hogs electrons and keeps them away from the Hstart text, H, end text atoms. This gives the oxygen end of the water molecule a partial negative charge, while the hydrogen end has a partial positive charge.
Q. Why does a positive partial charge appear on a hydrogen atom and a negative partial charge on an oxygen atom in a water molecule?
The slight positive charges on the hydrogen atoms in a water molecule attract the slight negative charges on the oxygen atoms of other water molecules. This tiny force of attraction is called a hydrogen bond. This bond is very weak.
Table of Contents
- Q. Why is oxygen partially negative and hydrogen positive?
- Q. Why does a positive partial charge appear on a hydrogen atom and a negative partial charge on an oxygen atom in a water molecule?
- Q. Why do the hydrogens bonded to oxygen have a much higher partial charge compared to the hydrogens bonded to carbon?
- Q. Why is the bond between the hydrogen and oxygen considered a polar covalent bond?
- Q. Is o2 a single or double bond?
- Q. What three shapes can carbon atoms bond?
- Q. How many types of bond can a carbon atom form?
- Q. Can carbon atoms form a quadruple bond?
Q. Why do the hydrogens bonded to oxygen have a much higher partial charge compared to the hydrogens bonded to carbon?
Since oxygen is more electronegative than hydrogen, the two bonds that are formed will be polar covalent, which means that a partial negative charge will be on the more electronegative atom – oxygen – and two partial positive charges will be on the less electronegative atoms – hydrogen.
Q. Why is the bond between the hydrogen and oxygen considered a polar covalent bond?
The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The shared electrons spend more time near the oxygen nucleus, giving it a small negative charge, than they spend near the hydrogen nuclei, giving these molecules a small positive charge.
Q. Is o2 a single or double bond?
The O2 Lewis structure has a double bond between two oxygen atoms.
Q. What three shapes can carbon atoms bond?
The three major types of covalent bonds are single, double, and triple bonds. A carbon atom can form the following bonds: Four single bonds. One double and two single bonds.
Q. How many types of bond can a carbon atom form?
four
Q. Can carbon atoms form a quadruple bond?
If we go on to the valence-bond model, in which bonds result from the overlap of atomic orbitals, we see a better explanation: carbon cannot form a quadruple bond because it doesn’t have enough atomic orbitals pointing in the right directions.