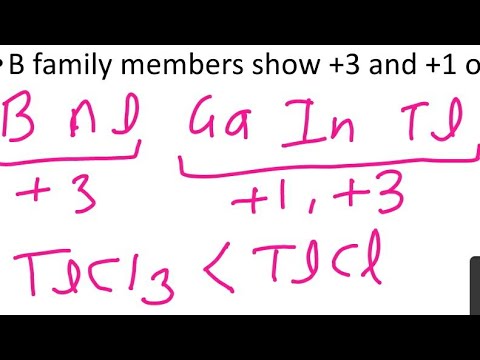Q. Why is the oxidation state of boron 3?
Due to its small size, removal of valence electrons of boron requires very high energy. Therefore, boron does not form +3 ions, but it forms covalent compounds. Boron is less electronegative than even hydrogen. So, compounds of boron contain boron in a positive oxidation state, generally +3.
Q. What is oxidation state of boron in nabh4?
+3
Table of Contents
- Q. Why is the oxidation state of boron 3?
- Q. What is oxidation state of boron in nabh4?
- Q. Why does boron show single oxidation?
- Q. What is the oxidation number of B in BH3?
- Q. What is the oxidation state of SiH4?
- Q. What is the oxidation state of hydrogen in SiH4?
- Q. What is the oxidation state of C in hco2h?
- Q. What is the oxidation number of oxygen in bao2?
- Q. What is oxidation number of Cr in Cr2O72?
- Q. What is the oxidation number for po4 3?
- Q. What is the oxidation number of Sulphur in sodium tetrathionate?
- Q. What is oxidation number of CN 6?
- Q. What is the oxidation number of fe4 Fe CN 6 3?
- Q. What is the chemical name of k3?
- Q. Are you pack name of k3fe CN 6?
- Q. What is FE CN 3?
- Q. What is the hybridization of fe4 Fe CN 6 3?
- Q. What is the hybridization of Fe CN 6 4?
- Q. What is the formula for Fe III Cl?
Q. Why does boron show single oxidation?
The +1 oxidation state becomes more stable as we go down the group. This is due to inert pair effect. In the case of last element, after the removal of one electron form p-orbital.
Q. What is the oxidation number of B in BH3?
Hydrogen has a -1 oxidation state in BH3. Therefore B is +3, each H is -1.
Q. What is the oxidation state of SiH4?
Oxidation number of Si in SiH4. This seems to be a double displacement (metathesis) reaction, where Si has oxidation state −4.
Q. What is the oxidation state of hydrogen in SiH4?
In hydrogen peroxide H2O2, the oxidation number of O is -1 and the range of the Oxidation number that O can have are from O to -2 can sometimes also attain the oxidation numbers +1 and +2. Hence, H2O2 can act as an oxidising as well as reducing agent.
Q. What is the oxidation state of C in hco2h?
C is using an oxidation state of 1 . This is the full list of oxidation states for this molecule.
Q. What is the oxidation number of oxygen in bao2?
-2
Q. What is oxidation number of Cr in Cr2O72?
+ 6
Q. What is the oxidation number for po4 3?
+5
Q. What is the oxidation number of Sulphur in sodium tetrathionate?
The oxidation states of middle sulphur atoms are 0 each and terminal sulphur is +5 each.
Q. What is oxidation number of CN 6?
Q. What is the oxidation number of fe4 Fe CN 6 3?
+2
Q. What is the chemical name of k3?
Potassium trioxalatoiridium (III)
Q. Are you pack name of k3fe CN 6?
potassium hexacyanoferrate(II)
Q. What is FE CN 3?
Iron cyanide
| PubChem CID | 115022 |
|---|---|
| Structure | Find Similar Structures |
| Molecular Formula | C3FeN3 |
| Synonyms | Ferric cyanide Iron cyanide iron tricyanide iron(III) cyanide Iron cyanide (Fe(CN)3) More… |
| Molecular Weight | 133.90 |
Q. What is the hybridization of fe4 Fe CN 6 3?
CN is a powerful field ligand and uses inner 3d-orbitals to form a low spin complex. Because there are 6 ligands, it has octahedral geometry. So the hybridization of the complex is d2sp3.
Q. What is the hybridization of Fe CN 6 4?
According to valence bond theory in [Fe(CN)6]4 – complex Fe+2 involves d2sp3 hybridization that is inner orbital complex having no unpaired electron and hence it is diamagnetic.
Q. What is the formula for Fe III Cl?
FeCl3