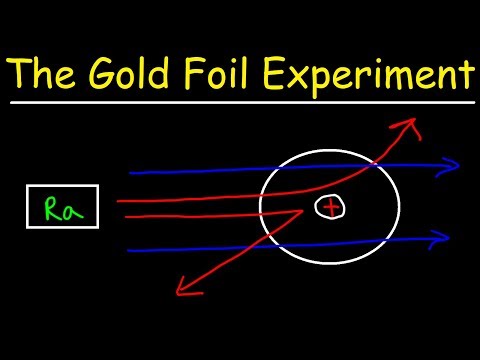
Gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. Since the gold foil was very thin, it was thought that the alpha particles could pass straight through it, or possibly puncture the foil. most of the alpha particles did pass straight through the foil.
Q. What does a Bohr model look like?
It is often called a “planetary” model, because it looks like the Sun with the electrons revolving around it like planets in their orbits. The orbits correspond to energy levels or shells. Thus, hydrogen (atomic number 1) has one electron in the K shell. Helium has two electrons in the K shell.
Table of Contents
Q. What did Rutherford’s model look like?
The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
Q. What is the conclusion of Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
Q. Why is it called the gold foil experiment?
Rutherford’s experiment is called the gold foil experiment because he used gold foil. 3. How did he know that an atom was mostly empty space? He knew that an atom was made of mostly empty space because most particles passed straight through the foil.
Q. How does the gold foil experiment work?
The Rutherford Gold Foil experiment shot minute particles at a thin sheet of gold. It was found that a small percentage of the particles were deflected, while a majority passed through the sheet. This caused Rutherford to conclude that the mass of an atom was concentrated at its center.
Q. Are gold atoms mainly empty space?
Atoms are mostly empty space. Because gold atoms are large and the foil was thin, he could be sure that his beam would pass through (if it did) just a few gold atoms. That means there are roughly five orders of magnitude of space between the nucleus and the nearest electron.